Korean J Physiol Pharmacol.
2008 Dec;12(6):337-342. 10.4196/kjpp.2008.12.6.337.
Diversity of Ion Channels in Human Bone Marrow Mesenchymal Stem Cells from Amyotrophic Lateral Sclerosis Patients
- Affiliations
-
- 1Division of Molecular and Life Sciences, Hanyang University, Ansan, 426-791, Korea.
- 2Department of Neurology, Hanyang University Hospital, Seoul 133-792, Korea.
- 3Bioengineering Institute, CoreStem Inc., Seoul 133-822, Korea.
- 4Department of Biomedical Engineering, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea.
- 5Department of Physiology, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea. yangmik@chungbuk.ac.kr
- KMID: 2285361
- DOI: http://doi.org/10.4196/kjpp.2008.12.6.337
Abstract
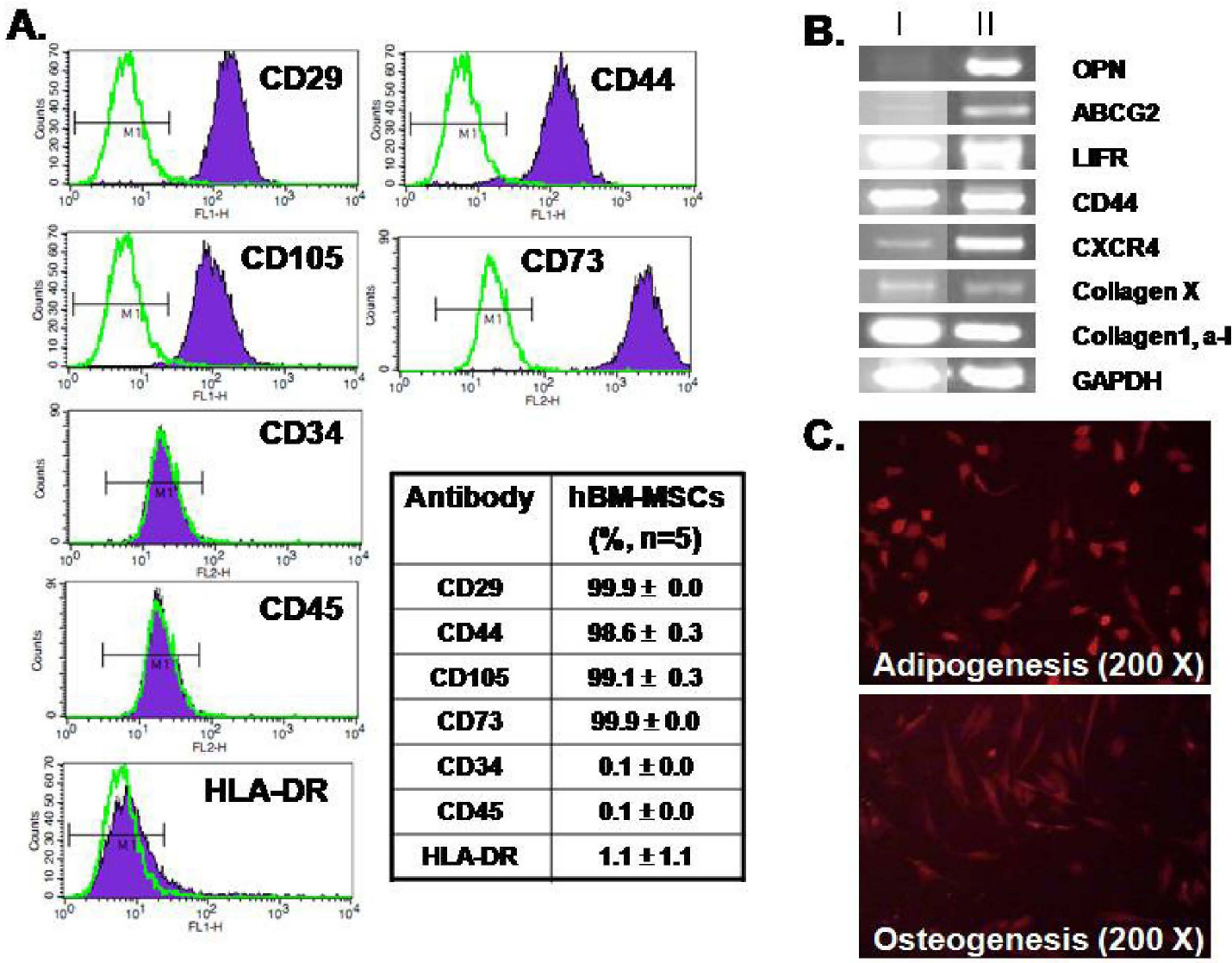
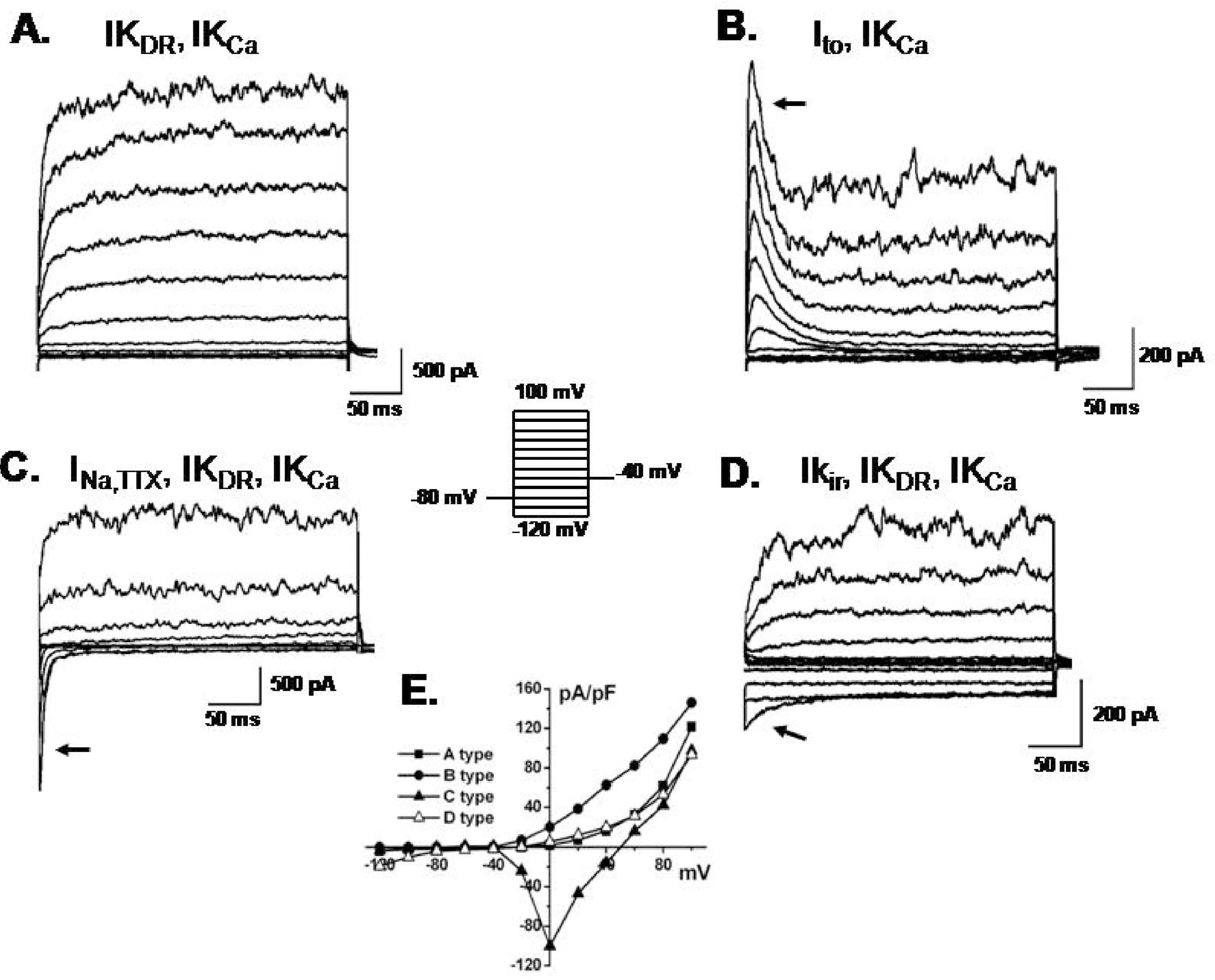
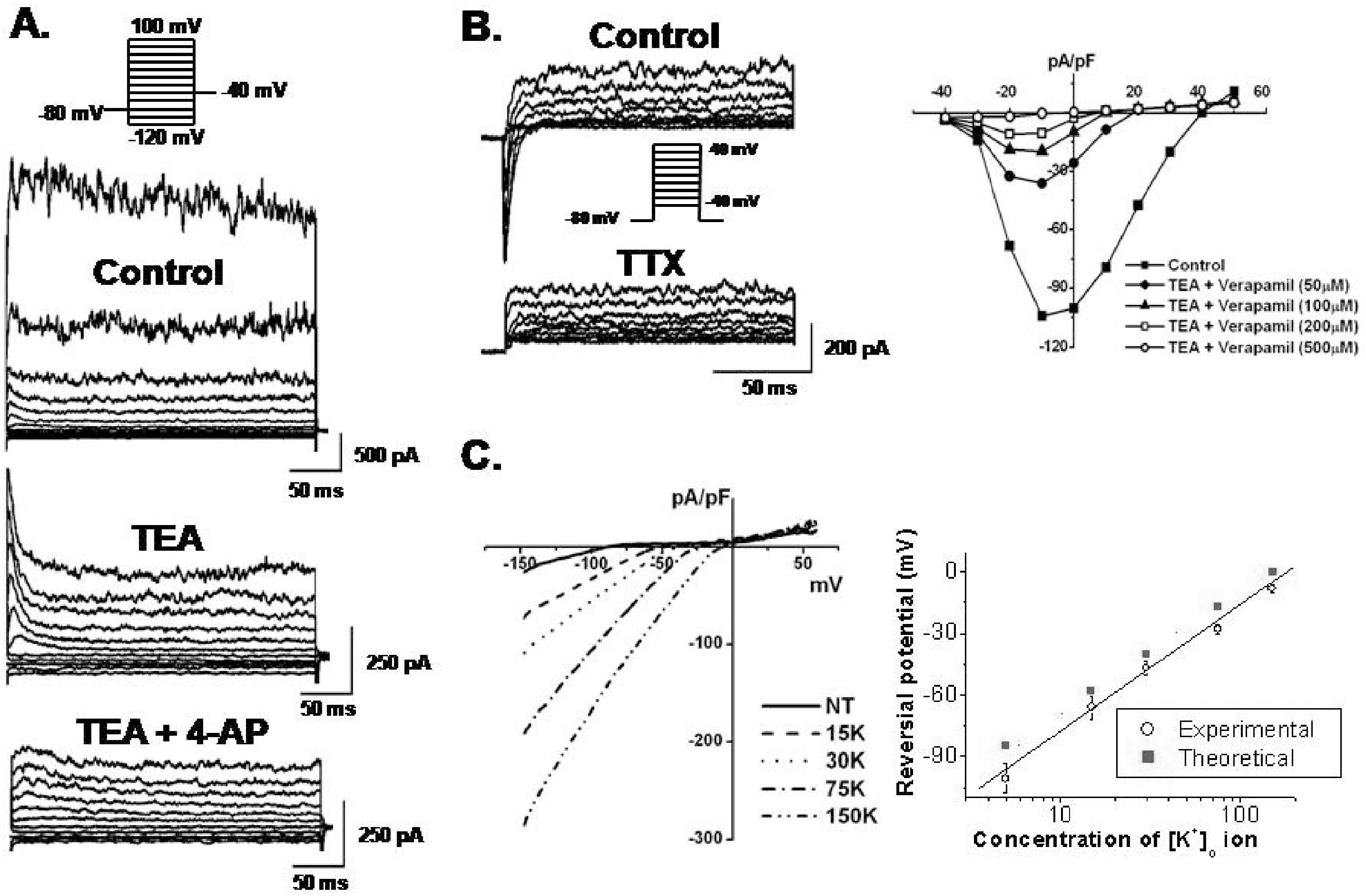
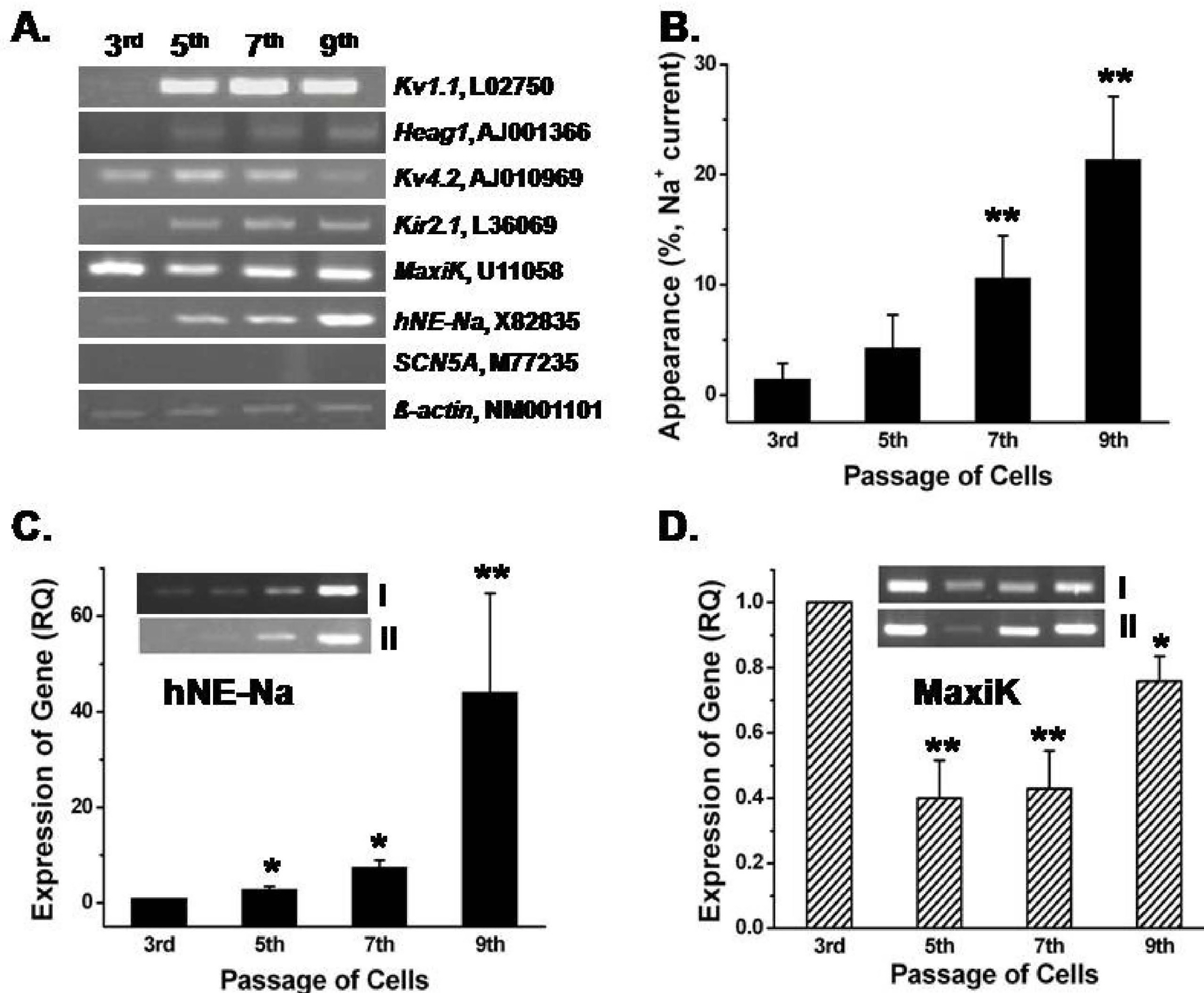
- Human bone marrow mesenchymal stem cells (hBM-MSCs) represent a potentially valuable cell type for clinical therapeutic applications. The present study was designed to evaluate the effect of long-term culturing (up to 10th passages) of hBM-MSCs from eight individual amyotrophic lateral sclerosis (ALS) patients, focusing on functional ion channels. All hBM-MSCs contain several MSCs markers with no significant differences, whereas the distribution of functional ion channels was shown to be different between cells. Four types of K+ currents, including noise-like Ca+2-activated K+ current (IKCa), a transient outward K+ current (Ito), a delayed rectifier K+ current (IKDR), and an inward-rectifier K+ current (Kir) were heterogeneously present in these cells, and a TTX-sensitive Na+ current (INa,TTX) was also recorded. In the RT-PCR analysis, Kv1.1, heag1, Kv4.2, Kir2.1, MaxiK, and hNE-Na were detected. In particular, INa,TTX showed a significant passage-dependent increase. This is the first report showing that functional ion channel profiling depend on the cellular passage of hBM-MSCs
Keyword
MeSH Terms
Figure
Reference
-
Balana B., Nicoletti C., Zahanich I., Graf EM., Christ T., Boxberger S., Ravens U. 5-Azacytidine induces changes in electrophysiological properties of human mesenchymal stem cells. Cell Res. 16:949–960. 2006.
ArticleBanfi A., Muraglia A., Dozin B., Mastrogiacomo M., Cancedda R., Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 28:707–715. 2000.Baxter MA., Wynn RF., Jowitt SN., Wraith JE., Fairbairn LJ., Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 22:675–682. 2004.
ArticleBiagiotti T., D'Amico M., Marzi I., Di Gennaro P., Arcangeli A., Wanke E., Olivotto M. Cell renewing in neuroblastoma: electrophy-siological and immunocytochemical characterization of stem cells and derivatives. Stem Cells. 24:443–453. 2006.
ArticleBonab MM., Alimoghaddam K., Talebian F., Ghaffari SH., Ghavamzadeh A., Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 7:14. 2006.
ArticleDeng XL., Lau CP., Lai K., Cheung KF., Lau GK., Li GR. Cell cycle-dependent expression of potassium channels and cell proliferation in rat mesenchymal stem cells from bone marrow. Cell Prolif. 40:656–670. 2007.
ArticleDeng XL., Sun HY., Lau CP., Li GR. Properties of ion channels in rabbit mesenchymal stem cells from bone marrow. Biochem Biophys Res Commun. 348:301–309. 2006.
ArticleFriedenstein AJ., Deriglasova UF., Kulagina NN., Panasuk AF., Rudakowa SF., Luria EA., Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 2:83–92. 1974.Hannouche D., Terai H., Fuchs JR., Terada S., Zand S., Nasseri BA., Petite H., Sedel L., Vacanti JP. Engineering of implantable cartilaginous structures from bone marrow-derived mesenchymal stem cells. Tissue Eng. 13:87–99. 2007.
ArticleKarnieli O., Izhar-Prato Y., Bulvik S., Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 25:2837–2844. 2007.
ArticleLi GR., Deng XL., Sun H., Chung SS., Tse HF., Lau CP. Ion channels in mesenchymal stem cells from rat bone marrow. Stem Cells. 24:1519–1528. 2006.
ArticleLi GR., Sun H., Deng X., Lau CP. Characterization of ionic currents in human mesenchymal stem cells from bone marrow. Stem Cells. 23:371–382. 2005.
ArticleMacFarlane SN., Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia. 30:39–48. 2000.
ArticleMazzini L., Mareschi K., Ferrero I., Vassallo E., Oliveri G., Boccaletti R., Testa L., Livigni S., Fagioli F. Autologous mesenchymal stem cells: clinical applications in amyotrophic lateral sclerosis. Neurol Res. 28:523–526. 2006.
ArticleOrlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson SM., Li B., Pickel J., McKay R., Nadal-Ginard B., Bodine DM., Leri A., Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 410:701–705. 2001.
ArticleOuadid-Ahidouch H., Roudbaraki M., Delcourt P., Ahidouch A., Joury N., Prevarskaya N. Functional and molecular identification of intermediate-conductance Ca2+-activated K+ channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol. 287:C125–134. 2004.Park KS., Jung KH., Kim SH., Kim KS., Choi MR., Kim Y., Chai YG. Functional expression of ion channels in mesenchymal stem cells derived from umbilical cord vein. Stem Cells. 25:2044–2052. 2007.
ArticleRombouts WJ., Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 17:160–170. 2003.
ArticleStenderup K., Justesen J., Clausen C., Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 33:919–926. 2003.
ArticleTao R., Lau CP., Tse HF., Li GR. Functional ion channels in mouse bone marrow mesenchymal stem cells. Am J Physiol Cell Physiol. 293:C1561–1567. 2007.
ArticleTomita S., Li RK., Weisel RD., Mickle DA., Kim EJ., Sakai T., Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 100(Suppl 19):II247–256. 1999.
ArticleWeiss ML., Medicetty S., Bledsoe AR., Rachakatla RS., Choi M., Merchav S., Luo Y., Rao MS., Velagaleti G., Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 24:781–792. 2006.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem Cell Therapy for Neurodegenerative Diseases
- Clinical Use of Mesenchymal Stem Cells in Bone Regeneration
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Amyotrophic Lateral Sclerosis Associated With CADASIL
- Efficacy and Safety of Autologous Bone Marrow-derived Mesenchymal Stem Cell Treatment in Patients With Amyotrophic Lateral Sclerosis