Korean J Pain.
2015 Apr;28(2):96-104. 10.3344/kjp.2015.28.2.96.
Anti-allodynic Efficacy of NMDA Antagonist Peptide and Noradrenaline Alone and in Combination in Rodent Neuropathic Pain Model
- Affiliations
-
- 1Physiology Research Center, Department of Physiology, Iran University of Medical Sciences, Tehran, Iran. nasirinezhad.f@iums.ac.ir
- 2Department of Physiology, Ilam University of Medical Sciences, Ilam, Iran.
- KMID: 2278266
- DOI: http://doi.org/10.3344/kjp.2015.28.2.96
Abstract
- BACKGROUND
The present experiment was conducted to identify the cooperative effect of serine histogranin (SHG) and noradrenaline in alleviating peripheral neuropathic pain.
METHODS
Chronic constriction injury of the right sciatic nerve was used to induce chronic neuropathic pain. For drug delivery, a PE10 tube was inserted into the subarachnoid space. Acetone drops and a 44degrees C water bath were used to evaluate the cold and heat allodynia, respectively. Placing and grasping reflexes were used to assess the locomotor system.
RESULTS
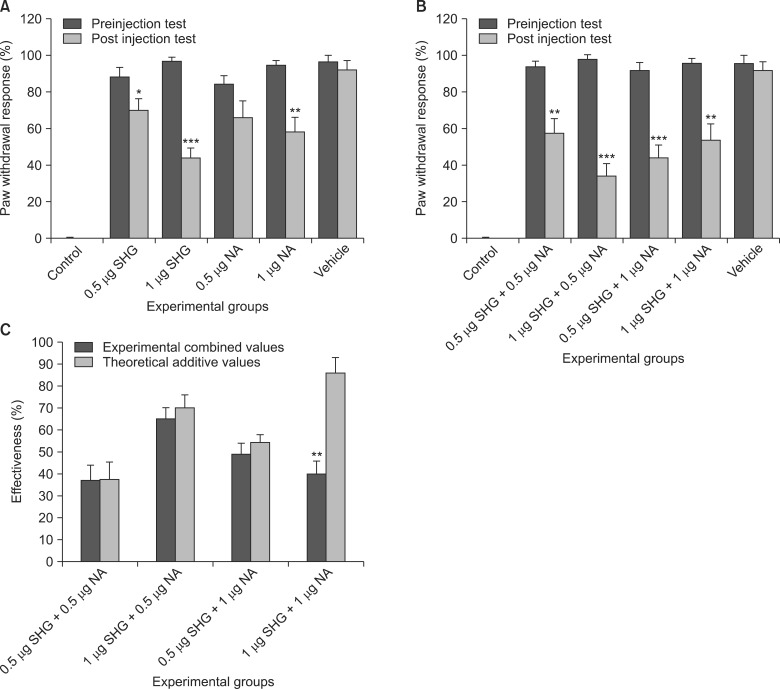
SHG at 0.5 and 1 microg significantly (P < 0.05) decreased the thermal allodynia. The cold allodynia was also significantly reduced by intrathecal injections of 0.5 (P < 0.05) and 1 microg (P < 0.001) of SHG. 1 microg of noradrenaline, but not 0.5 microg, significantly alleviated the cold (P < 0.01) and thermal (P < 0.05) allodynia. The ameliorating effect of noradrenaline or SHG disappeared when the two compounds were administrated in equal concentrations. A significant difference (P < 0.01 in the acetone and P < 0.05 in the heat) was observed in the groups under equal doses of the two compounds, with a lower effectiveness of the combination therapy.
CONCLUSIONS
Our findings suggest that the simultaneous administrations of noradrenaline and SHG do not result in synergistic analgesia, and combination therapy may not be a good approach to the treatment of chronic neuropathic pain syndrome.
Keyword
MeSH Terms
Figure
Reference
-
1. D'Angelo R, Morreale A, Donadio V, Boriani S, Maraldi N, Plazzi G, et al. Neuropathic pain following spinal cord injury: what we know about mechanisms, assessment and management. Eur Rev Med Pharmacol Sci. 2013; 17:3257–3261. PMID: 24338470.2. Hosseini M, Karami Z, Janzadenh A, Jameie SB, Haji Mashadi Z, Yousefifard M, et al. The effect of intrathecal administration of muscimol on modulation of neuropathic pain symptoms resulting from spinal cord injury; an experimental study. Emergency. 2014; 2:151–157.3. Okazaki R, Namba H, Yoshida H, Okai H, Taguchi K, Kawamura M. Combined antiallodynic effect of Neurotropin® and pregabalin in rats with L5-spinal nerve ligation. Life Sci. 2013; 92:259–265. PMID: 23333830.
Article4. NasiriNezhad F, Sagen J. NMDA antagonist peptide supplementation enhances pain alleviation by adrenal medullary transplants. Cell Transplant. 2005; 14:203–211. PMID: 15929555.
Article5. Nasiri Nejad F, Manaheji H. Sensory and motor behaviors of neuropathic rats following spinal transplantation of chromaffin cells. Razi J Med Sci. 2003; 9:581–592.6. Siegan JB, Sagen J. Attenuation of NMDA-induced spinal hypersensitivity by adrenal medullary transplants. Brain Res. 1995; 680:88–98. PMID: 7663988.
Article7. Siegan JB, Hama AT, Sagen J. Suppression of neuropathic pain by a naturally-derived peptide with NMDA antagonist activity. Brain Res. 1997; 755:331–334. PMID: 9175901.
Article8. Hahm TS, Ahn HJ, Ryu S, Gwak MS, Choi SJ, Kim JK, et al. Combined carbamazepine and pregabalin therapy in a rat model of neuropathic pain. Br J Anaesth. 2012; 109:968–974. PMID: 22936823.
Article9. Amin B, Hajhashemi V, Hosseinzadeh H, Abnous K. Antinociceptive evaluation of ceftriaxone and minocycline alone and in combination in a neuropathic pain model in rat. Neuroscience. 2012; 224:15–25. PMID: 22871519.
Article10. Hama A, Basler A, Sagen J. Enhancement of morphine antinociception with the peptide N-methyl-D-aspartate receptor antagonist [Ser1]-histogranin in the rat formalin test. Brain Res. 2006; 1095:59–64. PMID: 16712813.
Article11. Rojo ML, Rodríguez-Gaztelumendi A, Pazos Á, Díaz Á. Differential adaptive changes on serotonin and noradrenaline transporters in a rat model of peripheral neuropathic pain. Neurosci Lett. 2012; 515:181–186. PMID: 22480692.
Article12. Mladenova G, Annedi SC, Ramnauth J, Maddaford SP, Rakhit S, Andrews JS, et al. First-in-class, dual-action, 3,5-disubstituted indole derivatives having human nitric oxide synthase (nNOS) and norepinephrine reuptake inhibitory (NERI) activity for the treatment of neuropathic pain. J Med Chem. 2012; 55:3488–3501. PMID: 22420844.
Article13. Jiang LY, Li SR, Zhao FY, Spanswick D, Lin MT. Norepinephrine can act via alpha(2)-adrenoceptors to reduce the hyper-excitability of spinal dorsal horn neurons following chronic nerve injury. J Formos Med Assoc. 2010; 109:438–445. PMID: 20610145.
Article14. Tallarida RJ. Quantitative methods for assessing drug synergism. Genes Cancer. 2011; 2:1003–1008. PMID: 22737266.
Article15. Tallarida RJ. Revisiting the isobole and related quantitative methods for assessing drug synergism. J Pharmacol Exp Ther. 2012; 342:2–8. PMID: 22511201.
Article16. Nama S, Meenan DR, Fritz WT. The use of sub-anesthetic intravenous ketamine and adjuvant dexmedetomidine when treating acute pain from CRPS. Pain Physician. 2010; 13:365–368. PMID: 20648205.17. Romero TR, Guzzo LS, Perez AC, Klein A, Duarte ID. Noradrenaline activates the NO/cGMP/ATP-sensitive K(+) channels pathway to induce peripheral antinociception in rats. Nitric Oxide. 2012; 26:157–161. PMID: 22330728.
Article18. Crosby G, Russo MA, Szabo MD, Davies KR. Subarachnoid clonidine reduces spinal cord blood flow and glucose utilization in conscious rats. Anesthesiology. 1990; 73:1179–1185. PMID: 2248395.
Article19. Raiteri M. Functional pharmacology in human brain. Pharmacol Rev. 2006; 58:162–193. PMID: 16714485.
Article20. Parodi M, Patti L, Grilli M, Raiteri M, Marchi M. Nicotine has a permissive role on the activation of metabotropic glutamate 5 receptors coexisting with nicotinic receptors on rat hippocampal noradrenergic nerve terminals. Neurochem Int. 2006; 48:138–143. PMID: 16214264.
Article21. Alagarsamy S, Saugstad J, Warren L, Mansuy IM, Gereau RW 4th, Conn PJ. NMDA-induced potentiation of mGluR5 is mediated by activation of protein phosphatase 2B/calcineurin. Neuropharmacology. 2005; 49(Suppl 1):135–145. PMID: 16005030.
Article22. Luccini E, Musante V, Neri E, Brambilla Bas M, Severi P, Raiteri M, et al. Functional interactions between presynaptic NMDA receptors and metabotropic glutamate receptors co-expressed on rat and human noradrenergic terminals. Br J Pharmacol. 2007; 151:1087–1094. PMID: 17592518.
Article23. Pittaluga A, Pattarini R, Severi P, Raiteri M. Human brain N-methyl-D-aspartate receptors regulating noradrenaline release are positively modulated by HIV-1 coat protein gp120. AIDS. 1996; 10:463–468. PMID: 8724036.
Article24. Kamisaki Y, Hamada T, Maeda K, Ishimura M, Itoh T. Presynaptic alpha 2 adrenoceptors inhibit glutamate release from rat spinal cord synaptosomes. J Neurochem. 1993; 60:522–526. PMID: 8093480.
Article25. Nishiyama T, Gyermek L, Lee C, Kawasaki-Yatsugi S, Yamaguchi T, Hanaoka K. The analgesic interaction between intrathecal clonidine and glutamate receptor antagonists on thermal and formalin-induced pain in rats. Anesth Analg. 2001; 92:725–732. PMID: 11226109.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuropathic Pain Management with NMDA Receeptor Antagonist (Ketamine ) in Pain Clinic
- Intramuscular Injection of Ketamine in the Treatment of Postburn Neuropathic Pain: A case report
- Synergistic anti-allodynic effect between intraperitoneal thalidomide and morphine on rat spinal nerve ligation-induced neuropathic pain
- Anti-nociceptive effects of dual neuropeptide antagonist therapy in mouse model of neuropathic and inflammatory pain
- Effect of NMDA Receptor Antagonist and NOS Inhibitor on Spinal Sensory Neurons of Neuropathic Pain Animal Model