Korean J Nutr.
2011 Feb;44(1):5-15. 10.4163/kjn.2011.44.1.5.
Effects of Iron Overload during Pregnancy on Oxidative Stress in Maternal Rats
- Affiliations
-
- 1Department of Food and Nutrition & Research Institute of Human Ecology, Seoul National University, Seoul 151-742, Korea. lysook@snu.ac.kr
- KMID: 2268340
- DOI: http://doi.org/10.4163/kjn.2011.44.1.5
Abstract
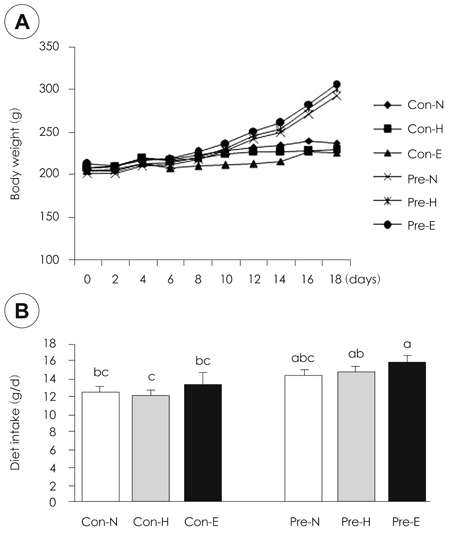
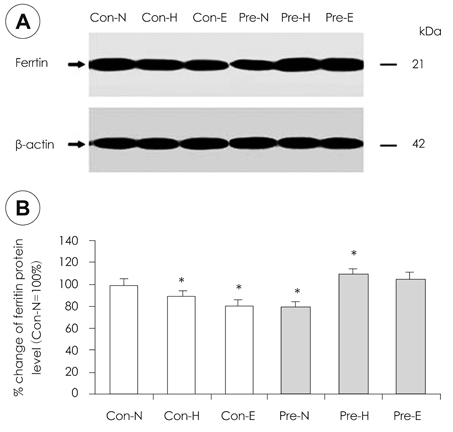
- Although iron is an essential mineral, excess iron intake during pregnancy may increase oxidative stress in tissues. This study was conducted to investigate the effects of iron overload during pregnancy on iron status and oxidative stress in maternal rats. Ten week-old female Sprague-Dawley rats were mated with male rats. Non-pregnant (control) and pregnant rats were fed diets containing normal Fe (35 mg/kg diet), high Fe (350 mg/kg diet), or excess Fe (1,050 mg/kg diet) during pregnancy. Rats were sacrificed on pregnancy day 19. No significant difference in weight gain, diet intake, or litter size was observed according to iron intake levels. Furthermore, serum iron, hemoglobin, and hematocrit were not different among the rats administered the three levels of Fe both in the control and pregnant groups. However, the iron levels were lower in pregnant rats than those in the control. The liver and spleen iron contents increased significantly in the excess Fe group. An increase in liver ferritin levels with increasing iron intake was observed. Protein carbonyl content, as a marker of oxidative stress, increased significantly in liver with increasing iron intake but not malondialdehyde. Glutathione peroxidase activity in the liver of pregnant rats fed excess iron decreased significantly. Bcl-2 protein expression in the liver declined remarkably with increasing maternal iron intake in pregnant rats. Taken together, iron overload during pregnancy had little effect on hematology. However, the deposits of iron in the liver and the decline in antioxidant enzyme activity implied increased oxidative stress in tissues of the excess Fe group. These results suggest that excess iron intake during pregnancy increases oxidative stress in maternal tissues and may also affect fetal tissues.
Keyword
MeSH Terms
Figure
Reference
-
1. Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001. 33(10):940–959.
Article2. Swanson CA. Iron intake and regulation: implications for iron deficiency and iron overload. Alcohol. 2003. 30(2):99–102.
Article3. Levenson CW, Tassabehji NM. Iron and ageing: an introduction to iron regulatory mechanisms. Ageing Res Rev. 2004. 3(3):251–263.
Article4. Ministry of Health & Welfare. 2008 the Report of Korean National Health & Nutrition Examination Survey. 2009.5. Cho JH, Ahn HS, Bae HS. The use of iron supplements of pregnant women and pregnancy outcome. Korean J Community Nutr. 2009. 14(3):327–339.6. Casanueva E, Viteri FE. Iron and oxidative stress in pregnancy. J Nutr. 2003. 133(5):Suppl 2. 1700S–1708S.
Article7. Southon S, Wright AJ, Fairweather-Tait SJ. The effect of differences in dietary iron intake on 59Fe absorption and duodenal morphology in pregnant rats. Br J Nutr. 1989. 62(3):707–717.
Article8. Barrett JFR, Whittaker PG, Williams JG, Lind T. Absorption of non-haem iron from food during normal pregnancy. BMJ. 1994. 309:79–82.
Article9. Long PJ. Rethinking iron supplementation during pregnancy. J Nurse Midwifery. 1995. 40(1):36–40.
Article10. Scholl TO. High third-trimester ferritin concentration: associations with very preterm delivery, infection, and maternal nutritional status. Obstet Gynecol. 1998. 92:161–166.
Article11. Kim YJ, Hong YC, Lee KH, Park HJ, Park EA, Moon HS, Ha EH. Oxidative stress in pregnant women and birth weight reduction. Reprod Toxicol. 2005. 19:487–492.
Article12. Atamer Y, Kocyigit Y, Yokus B, Atamer A, Erden AC. Lipid peroxidation, antioxidant defense, status of trace metals and leptin levels in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2005. 119(1):60–66.
Article13. Vanderlelie J, Venardos K, Clifton L, Gude NM, Clarke FM, Perkins AV. Increased Biological Oxidation and Reduced Anti-oxidant Enzyme Activity in Pre-eclamptic Placentae. Placenta. 2005. 26:53–58.
Article14. Sorenson CM. Bcl-2 family members and disease. Biochim Biophys Acta. 2004. 1644(2-3):169–177.
Article15. Motoyama S, Saito S, Saito R, Minamiya Y, Nakamura M, Okuyama M, Imano H, Ogawa J. Hydrogen peroxide-dependent declines in Bcl-2 induce apoptosis in hypoxic liver. J Surg Res. 2003. 110(1):211–216.
Article16. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993. 123:1939–1951.
Article17. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by Thiobarbituric acid reaction. Anal Biochem. 1979. 95:351–358.
Article18. Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn B, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990. 186:464–478.19. Aebi H. Catalase in Vitro. Methods Enzymol. 1984. 105:121–126.20. Kochanowski BA, Sherman AR. Iron status of suckling rats as influenced by maternal diet during gestation and lactation. Br J Nutr. 1983. 49(1):51–57.
Article21. Southon S, Wright AJ, Fairweather-Tait SJ. The effect of differences in dietary iron intake on 59Fe absorption and duodenal morphology in pregnant rats. Br J Nutr. 1989. 62(3):707–717.
Article22. Finch CA, Huebers HA, Miller LR, Josephson BM, Shepard TH, Mackler B. Fetal iron balance in the rat. Am J Clin Nutr. 1983. 37(6):910–917.
Article23. Sochaski MA, Bartfay WJ, Thorpe SR, Baynes JW, Bartfay E, Lehotay DC, Liu PP. Lipid peroxidation and protein modification in a mouse model of chronic iron overload. Metabolism. 2002. 51(5):645–651.
Article24. Lin WJ, Kirksey A. Effects of different levels of dietary iron on pregnancy superimposed upon growth in the rat. J Nutr. 1976. 106:543–554.
Article25. Tran T, Wax JR, Philput C, Steinfeld JD, Ingardia CJ. Intentional iron overdose in pregnancymanagement and outcome. J Emerg Med. 2000. 18(2):225–228.
Article26. Van den Broek N. Anemia and micronutrient deficiencies. Br Med Bull. 2003. 67:149–160.27. Kang BH, Son HY, Ha CS, Lee HS, Song SW. Reference Values of Hematology and Serum Chemistry in Ktc: Sprague-Dawely Rats. Korean J Lab Anim Sci. 1995. 11(2):141–145.28. Nair KM, Bhaskaram P, Balakrishna N, Ravinder P, Sesikeran B. Response of hemoglobin, serum ferritin, and serum transferrin receptor during iron supplementation in pregnancy: A prospective study. Nutrition. 2004. 20(10):896–899.
Article29. Lee JI, Kang SA, Kim SK, Lim HS. A cross sectional study of maternal iron status of Korean women during pregnancy. Nutr Res. 2002. 22(12):1377–1388.
Article30. Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. Am J Clin Nutr. 2003. 78:773–781.
Article31. Sherman AR, Moran PE. Copper metabolism in iron-deficient maternal and neonatal rats. J Nutr. 1984. 114(2):298–306.
Article32. Bashiri A, Burstein E, Sheiner E, Mazor M. Anemia during pregnancy and treatment with intravenous iron: review of the literature. Eur J Obstet Gynecol Reprod Biol. 2003. 110:2–7.
Article33. Bresgen N, Jaksch H, Lacher H, Ohlenschlager I, Uchida K, Eckl PM. Iron-mediated oxidative stress plays an essential role in ferritin-induced cell death. Free Radic Biol Med. 2010. 48:1347–1357.
Article34. Templeton DM, Liu Y. Genetic regulation of cell function in response to iron overload or chelation. Biochim Biophys Acta. 2003. 1619(2):113–124.
Article35. Khan MF, Wu X, Tipnis UR, Ansari GA, Boor PJ. Protein adducts of malondialdehyde and 4-hydroxynonenal in livers of iron loaded rats: quantitation and localization. Toxicology. 2002. 173(3):193–201.
Article36. Knutson MD, Walter PB, Ames BN, Viteri FE. Both iron deficiency and daily iron supplements increase lipid peroxidation in rats. J Nutr. 2000. 130(3):621–628.
Article37. Srigiridhar K, Nair KM. Iron-deficient intestine is more susceptible to peroxidative damage during iron supplementation in rats. Free Radic Biol Med. 1998. 25(6):660–665.
Article38. Brady HJ, Gil-Gomez G. Bax: the pro-apoptotic Bcl-2 family member. Int J Biochem Cell Biol. 1998. 30(6):647–650.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Iron Supplementation in Experimental Hyperthyroidism: Effects on Oxidative Stress in Skeletal Muscle Tissue
- Effects of Maternal Anemia on the Iron Status of the Cord Blood and Pregnancy Outcomes
- Iron Deficiency Anemia in Pregnancy
- Associations between Lifestyle Factors and Iron Overload in Korean Adults
- A Longitudinal Study on Maternal Iron and Folate Status During and After Pregnancy in Korean Women