Ann Dermatol.
2013 Nov;25(4):423-427. 10.5021/ad.2013.25.4.423.
Decreased Expression of Type 5 17beta-Hydroxysteroid Dehydrogenase (AKR1C3) Protein Identified in Human Diabetic Skin Tissue
- Affiliations
-
- 1Department of Dermatology, Soonchunhyang University College of Medicine, Seoul, Korea. mkcho2001@hanmail.net
- KMID: 2265048
- DOI: http://doi.org/10.5021/ad.2013.25.4.423
Abstract
- BACKGROUND
Diabetes is characterized by chronic hyperglycemia, and hyperglycemia can increase reactive oxygen species (ROS) production from the mitochondrial electron transport chain. The formation of ROS in cells induces oxidative stress and activates oxidative damage-inducing genes. There is no research on the protein levels of oxidative damage-related genes AKR1C3 in human diabetic skin. We explored the expression of AKR1C3 in diabetic skin compared with normal skin tissue.
OBJECTIVE
To compare the expression of AKR1C3 in normal skin versus diabetic skin.
METHODS
AKR1C3 expression was evaluated by western blotting in 6 diabetic skin tissue samples and 6 normal skin samples. Immunohistochemical staining was carried out to analyze AKR1C3 expression in the 6 diabetic skin tissue samples (July 2009 to December 2011; Department of Plastic and Reconstructive Surgery at Soonchunhyang University Seoul Hospital, Seoul, Korea).
RESULTS
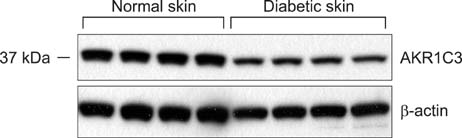
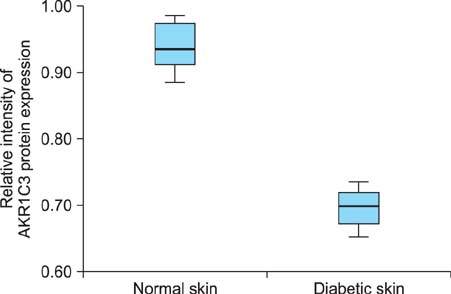
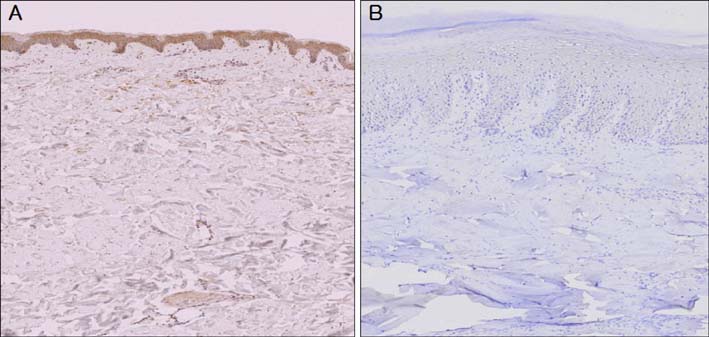
The western blotting showed a significant reduction in AKR1C3 protein expression in diabetic skin tissue compared to normal tissue. Immunohistochemical examination of AKR1C3 showed that it was weakly expressed in all diabetic skin samples.
CONCLUSION
We believe that AKR1C3 is related to diabetic skin in altered metabolic states which elevate ROS production.
MeSH Terms
Figure
Reference
-
1. Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab. 2000; 26:163–176.2. West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000; 17:171–180.
Article3. Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979; 59:527–605.
Article4. Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993; 62:797–821.
Article5. Farber JM, Levine RL. Sequence of a peptide susceptible to mixed-function oxidation. Probable cation binding site in glutamine synthetase. J Biol Chem. 1986; 261:4574–4578.
Article6. Climent I, Tsai L, Levine RL. Derivatization of gamma-lutamyl semialdehyde residues in oxidized proteins by fluoresceinamine. Anal Biochem. 1989; 182:226–232.
Article7. Levine RL. Oxidative modification of glutamine synthetase. I. Inactivation is due to loss of one histidine residue. J Biol Chem. 1983; 258:11823–11827.
Article8. Ellis EM. Reactive carbonyls and oxidative stress: potential for therapeutic intervention. Pharmacol Ther. 2007; 115:13–24.
Article9. Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. Biochem J. 1987; 245:243–250.
Article10. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414:813–820.
Article11. Brownlee M, Cerami A. The biochemistry of the complications of diabetes mellitus. Annu Rev Biochem. 1981; 50:385–432.
Article12. Brownlee M. Negative consequences of glycation. Metabolism. 2000; 49:2 Suppl 1. 9–13.
Article13. Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991; 10:339–352.
Article14. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002; 82:47–95.
Article15. Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000; 28:463–499.
Article16. Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991; 40:405–412.
Article17. Davies KJ, Delsignore ME, Lin SW. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J Biol Chem. 1987; 262:9902–9907.
Article18. Uchida K, Kawakishi S. Site-specific oxidation of angiotensin I by copper(II) and L-ascorbate: conversion of histidine residues to 2-imidazolones. Arch Biochem Biophys. 1990; 283:20–26.
Article19. Heinecke JW, Li W, Daehnke HL 3rd, Goldstein JA. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J Biol Chem. 1993; 268:4069–4077.
Article20. Chevion M, Berenshtein E, Stadtman ER. Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radic Res. 2000; 33:Suppl. S99–S108.21. Domínguez C, Ruiz E, Gussinye M, Carrascosa A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care. 1998; 21:1736–1742.
Article22. Cakatay U, Telci A, Salman S, Satman L, Sivas A. Oxidative protein damage in type I diabetic patients with and without complications. Endocr Res. 2000; 26:365–379.
Article23. Telci A, Cakatay U, Salman S, Satman I, Sivas A. Oxidative protein damage in early stage Type 1 diabetic patients. Diabetes Res Clin Pract. 2000; 50:213–223.
Article24. Jang YY, Song JH, Shin YK, Han ES, Lee CS. Protective effect of boldine on oxidative mitochondrial damage in streptozotocin-induced diabetic rats. Pharmacol Res. 2000; 42:361–371.
Article25. Cederberg J, Basu S, Eriksson UJ. Increased rate of lipid peroxidation and protein carbonylation in experimental diabetic pregnancy. Diabetologia. 2001; 44:766–774.
Article26. Altomare E, Grattagliano I, Vendemaile G, Micelli-Ferrari T, Signorile A, Cardia L. Oxidative protein damage in human diabetic eye: evidence of a retinal participation. Eur J Clin Invest. 1997; 27:141–147.
Article27. Dean RT, Fu S, Stocker R, Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 1997; 324:1–18.
Article28. Jez JM, Bennett MJ, Schlegel BP, Lewis M, Penning TM. Comparative anatomy of the aldo-keto reductase superfamily. Biochem J. 1997; 326:625–636.
Article29. Palackal NT, Lee SH, Harvey RG, Blair IA, Penning TM. Activation of polycyclic aromatic hydrocarbon trans-dihydrodiol proximate carcinogens by human aldo-keto reductase (AKR1C) enzymes and their functional overexpression in human lung carcinoma (A549) cells. J Biol Chem. 2002; 277:24799–24808.
Article30. Matsunaga T, Shintani S, Hara A. Multiplicity of mammalian reductases for xenobiotic carbonyl compounds. Drug Metab Pharmacokinet. 2006; 21:1–18.
Article31. Haber CA, Lam TK, Yu Z, Gupta N, Goh T, Bogdanovic E, et al. N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: possible role of oxidative stress. Am J Physiol Endocrinol Metab. 2003; 285:E744–E753.
Article32. Paolisso G, Giugliano D. Oxidative stress and insulin action: is there a relationship? Diabetologia. 1996; 39:357–363.
Article33. Ceriello A. Oxidative stress and glycemic regulation. Metabolism. 2000; 49:27–29.
Article34. Jin Y, Penning TM. Aldo-keto reductases and bioactivation/etoxication. Annu Rev Pharmacol Toxicol. 2007; 47:263–292.35. Hyndman D, Bauman DR, Heredia VV, Penning TM. The aldo-keto reductase superfamily homepage. Chem Biol Interact. 2003; 143-144:621–631.
Article36. O'connor T, Ireland LS, Harrison DJ, Hayes JD. Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. Biochem J. 1999; 343:487–504.37. Burczynski ME, Sridhar GR, Palackal NT, Penning TM. The reactive oxygen species--and Michael acceptor-inducible human aldo-keto reductase AKR1C1 reduces the alpha, etansaturated aldehyde 4-hydroxy-2-nonenal to 1,4-dihydroxy-2-onene. J Biol Chem. 2001; 276:2890–2897.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of AKR1C3 Protein in Human Keloid Skin Tissue
- 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibition Attenuates the Adverse Effects of Glucocorticoids on Dermal Papilla Cells
- Elevated Aurora Kinase A Protein Expression in Diabetic Skin Tissue
- Expression of Extracellular Superoxide Dismutase Protein in Diabetes
- Mutational Analysis of 17beta-hydroxysteroid dehydrogenase type 2 gene in Breast Cancers