Anat Cell Biol.
2013 Jun;46(2):85-92. 10.5115/acb.2013.46.2.85.
Biocompatability of carbon nanotubes with stem cells to treat CNS injuries
- Affiliations
-
- 1Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea. jelee@yuhs.ac
- 2Institute of Tissue Engineering (ITREN), Dankook University, Cheonan, Korea.
- 3Department of Anatomy, Dankook University College of Medicine, Cheonan, Korea.
- 4Department of Orthopedics, Brown University, Providence, RI, USA.
- 5BK 21 Project for Brain Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2263099
- DOI: http://doi.org/10.5115/acb.2013.46.2.85
Abstract
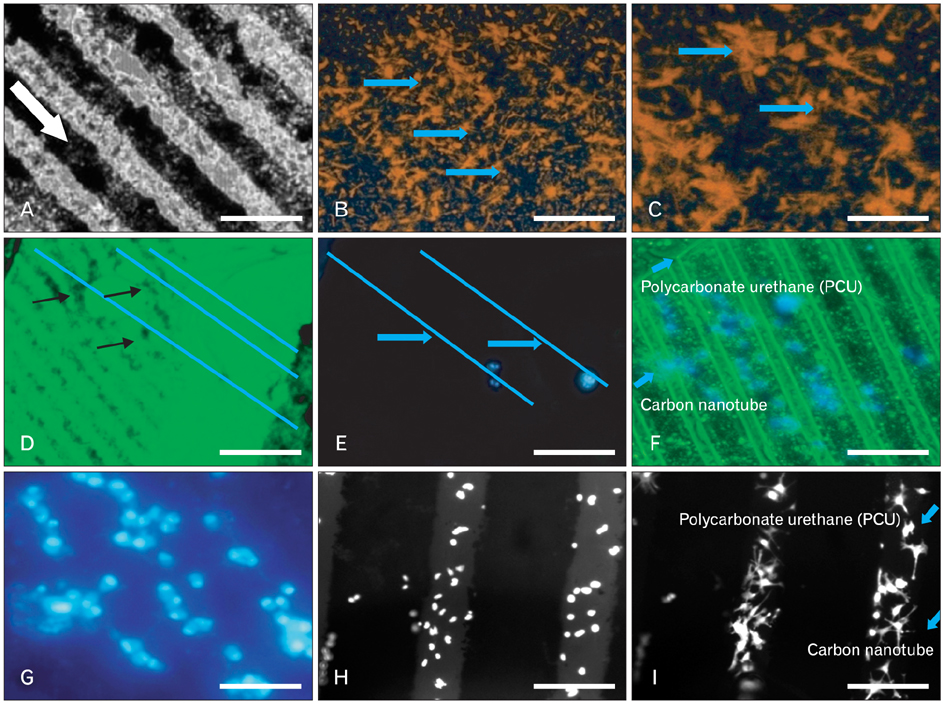
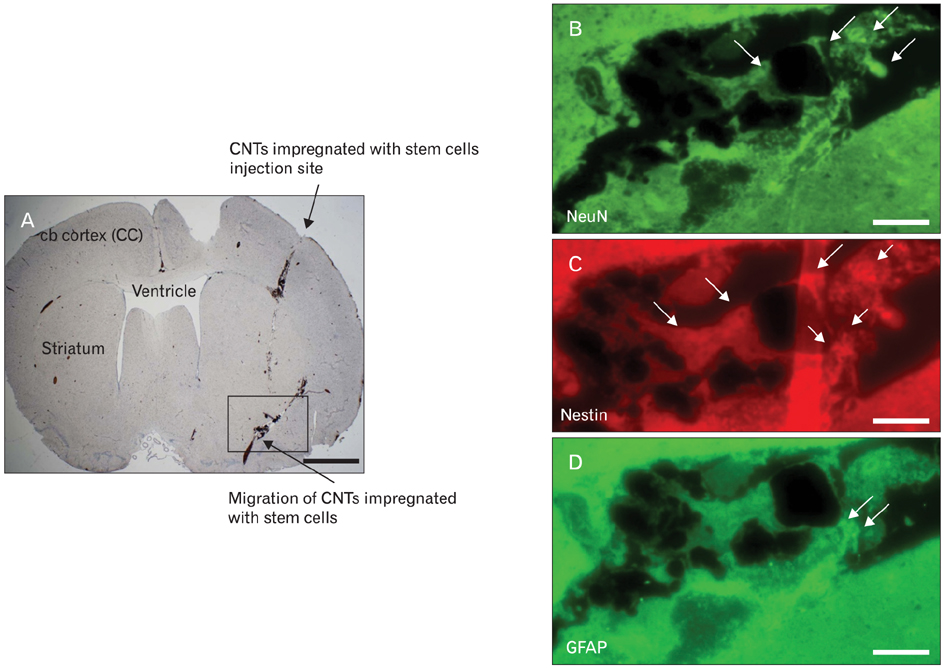
- Cases reporting traumatic injuries to the brain and spinal cord are extended range of disorders that affect a large percentage of the world's population. But, there are only few effective treatments available for central nervous system (CNS) injuries because the CNS is refractory to axonal regeneration and relatively inaccessible to many pharmacological treatments. The use of stem cell therapy in regenerative medicine has been extensively examined to replace lost cells during CNS injuries. But, given the complexity of CNS injuries oxidative stress, toxic byproducts, which prevails in the microenvironment during the diseased condition, may limit the survival of the transplanted stem cells affecting tissue regeneration and even longevity. Carbon nanotubes (CNT) are a new class of nanomaterials, which have been shown to be promising in different areas of nanomedicine for the prevention, diagnosis and therapy of certain diseases, including CNS diseases. In particular, the use of CNTs as substrates/scaffolds for supporting the stem cell differentiation has been an area of active research. Single-walled and multi-walled CNT's have been increasingly used as scaffolds for neuronal growth and more recently for neural stem cell growth and differentiation. This review summarizes recent research on the application of CNT-based materials to direct the differentiation of progenitor and stem cells toward specific neurons and to enhance axon regeneration and synaptogenesis for the effective treatment of CNS injuries. Nonetheless, accumulating data support the use of CNTs as a biocompatible and permissive substrate/scaffold for neural cells and such application holds great potential in neurological research.
Keyword
MeSH Terms
Figure
Reference
-
1. Gilmore JL, Yi X, Quan L, Kabanov AV. Novel nanomaterials for clinical neuroscience. J Neuroimmune Pharmacol. 2008. 3:83–94.2. Modi G, Pillay V, Choonara YE, Ndesendo VM, du Toit LC, Naidoo D. Nanotechnological applications for the treatment of neurodegenerative disorders. Prog Neurobiol. 2009. 88:272–285.3. Akerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease. J Neurosci. 2001. 21:8108–8118.4. Chai C, Leong KW. Biomaterials approach to expand and direct differentiation of stem cells. Mol Ther. 2007. 15:467–480.5. Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002. 99:3024–3029.6. Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002. 20:1111–1117.7. Chun YS, Byun K, Lee B. Induced pluripotent stem cells and personalized medicine: current progress and future perspectives. Anat Cell Biol. 2011. 44:245–255.8. Ourednik V, Ourednik J, Flax JD, Zawada WM, Hutt C, Yang C, Park KI, Kim SU, Sidman RL, Freed CR, Snyder EY. Segregation of human neural stem cells in the developing primate forebrain. Science. 2001. 293:1820–1824.9. Steindler DA. Neural stem cells, scaffolds, and chaperones. Nat Biotechnol. 2002. 20:1091–1093.10. Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995. 374:367–370.11. Martínez-Serrano A, Björklund A. Protection of the neostriatum against excitotoxic damage by neurotrophin-producing, genetically modified neural stem cells. J Neurosci. 1996. 16:4604–4616.12. Snyder EY, Yoon C, Flax JD, Macklis JD. Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc Natl Acad Sci U S A. 1997. 94:11663–11668.13. Gage FH. Cell therapy. Nature. 1998. 392:6679 Suppl. 18–24.14. Ma W, Fitzgerald W, Liu QY, O'Shaughnessy TJ, Maric D, Lin HJ, Alkon DL, Barker JL. CNS stem and progenitor cell differentiation into functional neuronal circuits in three-dimensional collagen gels. Exp Neurol. 2004. 190:276–288.15. Bellamkonda R, Ranieri JP, Bouche N, Aebischer P. Hydrogel-based three-dimensional matrix for neural cells. J Biomed Mater Res. 1995. 29:663–671.16. Woerly S, Petrov P, Syková E, Roitbak T, Simonová Z, Harvey AR. Neural tissue formation within porous hydrogels implanted in brain and spinal cord lesions: ultrastructural, immunohistochemical, and diffusion studies. Tissue Eng. 1999. 5:467–488.17. Zhang L, Webster TJ. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009. 4:66–80.18. Ilie I, Ilie R, Mocan T, Bartos D, Mocan L. Influence of nanomaterials on stem cell differentiation: designing an appropriate nanobiointerface. Int J Nanomedicine. 2012. 7:2211–2225.19. Lock J, Liu H. Nanomaterials enhance osteogenic differentiation of human mesenchymal stem cells similar to a short peptide of BMP-7. Int J Nanomedicine. 2011. 6:2769–2777.20. Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009. 8:543–557.21. Orza A, Soritau O, Olenic L, Diudea M, Florea A, Rus Ciuca D, Mihu C, Casciano D, Biris AS. Electrically conductive gold-coated collagen nanofibers for placental-derived mesenchymal stem cells enhanced differentiation and proliferation. ACS Nano. 2011. 5:4490–4503.22. Cellot G, Cilia E, Cipollone S, Rancic V, Sucapane A, Giordani S, Gambazzi L, Markram H, Grandolfo M, Scaini D, Gelain F, Casalis L, Prato M, Giugliano M, Ballerini L. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat Nanotechnol. 2009. 4:126–133.23. Georgakilas V, Kordatos K, Prato M, Guldi DM, Holzinger M, Hirsch A. Organic functionalization of carbon nanotubes. J Am Chem Soc. 2002. 124:760–761.24. Tasis D, Tagmatarchis N, Bianco A, Prato M. Chemistry of carbon nanotubes. Chem Rev. 2006. 106:1105–1136.25. Shvartzman-Cohen R, Levi-Kalisman Y, Nativ-Roth E, Yerushalmi-Rozen R. Generic approach for dispersing single-walled carbon nanotubes: the strength of a weak interaction. Langmuir. 2004. 20:6085–6088.26. Moore VC, Strano MS, Haroz EH, Hauge RH, Smalley RE, Schmidt J, Talmon Y. Individually suspended single-walled carbon nanotubes in various surfactants. Nano Lett. 2003. 3:1379–1382.27. Dieckmann GR, Dalton AB, Johnson PA, Razal J, Chen J, Giordano GM, Muñoz E, Musselman IH, Baughman RH, Draper RK. Controlled assembly of carbon nanotubes by designed amphiphilic Peptide helices. J Am Chem Soc. 2003. 125:1770–1777.28. Zheng M, Jagota A, Semke ED, Diner BA, McLean RS, Lustig SR, Richardson RE, Tassi NG. DNA-assisted dispersion and separation of carbon nanotubes. Nat Mater. 2003. 2:338–342.29. Matsumoto K, Sato C, Naka Y, Kitazawa A, Whitby RL, Shimizu N. Neurite outgrowths of neurons with neurotrophin-coated carbon nanotubes. J Biosci Bioeng. 2007. 103:216–220.30. Gheith MK, Sinani VA, Wicksted JP, Matts RL, Kotov NA. Single-walled carbon nanotube polyelectrolyte multilayers and freestanding films as a biocompatible platform for neuroprosthetic implants. Adv Mater. 2005. 17:2663–2670.31. Lovat V, Pantarotto D, Lagostena L, Cacciari B, Grandolfo M, Righi M, Spalluto G, Prato M, Ballerini L. Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett. 2005. 5:1107–1110.32. McKenzie JL, Waid MC, Shi R, Webster TJ. Decreased functions of astrocytes on carbon nanofiber materials. Biomaterials. 2004. 25:1309–1317.33. Nho Y, Kim JY, Khang D, Webster TJ, Lee JE. Adsorption of mesenchymal stem cells and cortical neural stem cells on carbon nanotube/polycarbonate urethane. Nanomedicine (Lond). 2010. 5:409–417.34. Kim JY, Khang D, Lee JE, Webster TJ. Decreased macrophage density on carbon nanotube patterns on polycarbonate urethane. J Biomed Mater Res A. 2009. 88:419–426.35. Lee JE, Khang D, Kim YE, Webster TJ. Stem cell impregnated carbon nanofibers/nanotubes for healing damaged neural tissue. Mater Res Soc Symp Proc. 2006. 915:0915-R01-07.36. Grinnell F, Feld MK. Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum-containing medium. J Biol Chem. 1982. 257:4888–4893.37. Horbett TA, Schway MB. Correlations between mouse 3T3 cell spreading and serum fibronectin adsorption on glass and hydroxyethylmethacrylate-ethylmethacrylate copolymers. J Biomed Mater Res. 1988. 22:763–793.38. Steele JG, Johnson G, Underwood PA. Role of serum vitronectin and fibronectin in adhesion of fibroblasts following seeding onto tissue culture polystyrene. J Biomed Mater Res. 1992. 26:861–884.39. Moon SU, Kim J, Bokara KK, Kim JY, Khang D, Webster TJ, Lee JE. Carbon nanotubes impregnated with subventricular zone neural progenitor cells promotes recovery from stroke. Int J Nanomedicine. 2012. 7:2751–2765.40. Khang D, Kim SY, Liu-Snyder P, Palmore GT, Durbin SM, Webster TJ. Enhanced fibronectin adsorption on carbon nanotube/poly(carbonate) urethane: independent role of surface nano-roughness and associated surface energy. Biomaterials. 2007. 28:4756–4768.41. Sorkin R, Greenbaum A, David-Pur M, Anava S, Ayali A, Ben-Jacob E, Hanein Y. Process entanglement as a neuronal anchorage mechanism to rough surfaces. Nanotechnology. 2009. 20:015101.42. Zhang X, Prasad S, Niyogi S, Morgan A, Ozkan M, Ozkan CS. Guided neurite growth on patterned carbon nanotubes. Sens Actuators B Chem. 2005. 106:843–850.43. Bekyarova E, Haddon RC, Parpura V. Kumar CS, editor. Biofunctionalization of carbon nanotubes. Biofunctionalization of Nanomaterials (Nanotechnologies for the Life Sciences). 2005. Vol. 1. Weinheim: Wiley;41–65.44. Bekyarova E, Ni Y, Malarkey EB, Montana V, McWilliams JL, Haddon RC, Parpura V. Applications of carbon nanotubes in biotechnology and biomedicine. J Biomed Nanotechnol. 2005. 1:3–17.45. Malarkey EB, Parpura V. Carbon nanotubes in neuroscience. Acta Neurochir Suppl. 2010. 106:337–341.46. Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neurosci. 2000. 14:175–182.47. Kubinová S, Syková E. Nanotechnologies in regenerative medicine. Minim Invasive Ther Allied Technol. 2010. 19:144–156.48. Hu H, Ni Y, Montana V, Haddon RC, Parpura V. Chemically Functionalized Carbon Nanotubes as Substrates for Neuronal Growth. Nano Lett. 2004. 4:507–511.49. Liopo AV, Stewart MP, Hudson J, Tour JM, Pappas TC. Biocompatibility of native and functionalized single-walled carbon nanotubes for neuronal interface. J Nanosci Nanotechnol. 2006. 6:1365–1374.50. Galvan-Garcia P, Keefer EW, Yang F, Zhang M, Fang S, Zakhidov AA, Baughman RH, Romero MI. Robust cell migration and neuronal growth on pristine carbon nanotube sheets and yarns. J Biomater Sci Polym Ed. 2007. 18:1245–1261.51. Dubin RA, Callegari G, Kohn J, Neimark A. Carbon nanotube fibers are compatible with Mammalian cells and neurons. IEEE Trans Nanobioscience. 2008. 7:11–14.52. Gabay T, Jakobs E, Ben-Jacob E, Hanein Y. Engineered self-organization of neural networks using carbon nanotube clusters. Phys A. 2005. 350:611–621.53. Sorkin R, Gabay T, Blinder P, Baranes D, Ben-Jacob E, Hanein Y. Compact self-wiring in cultured neural networks. J Neural Eng. 2006. 3:95–101.54. Jan E, Kotov NA. Successful differentiation of mouse neural stem cells on layer-by-layer assembled single-walled carbon nanotube composite. Nano Lett. 2007. 7:1123–1128.55. Kam NW, Jan E, Kotov NA. Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett. 2009. 9:273–278.56. Roman JA, Niedzielko TL, Haddon RC, Parpura V, Floyd CL. Single-walled carbon nanotubes chemically functionalized with polyethylene glycol promote tissue repair in a rat model of spinal cord injury. J Neurotrauma. 2011. 28:2349–2362.57. Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, Alexander A. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006. 92:5–22.58. Pacurari M, Castranova V, Vallyathan V. Single- and multi-wall carbon nanotubes versus asbestos: are the carbon nanotubes a new health risk to humans? J Toxicol Environ Health A. 2010. 73:378–395.59. Lacerda L, Bianco A, Prato M, Kostarelos K. Carbon nanotubes as nanomedicines: from toxicology to pharmacology. Adv Drug Deliv Rev. 2006. 58:1460–1470.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Carbon Monoxide in Neurovascular Repair Processing
- Effect of aspect ratio on the uptake and toxicity of hydroxylated-multi walled carbon nanotubes in the nematode, Caenorhabditis elegans
- Neural Stem Cells
- Effect of Multi-Walled Carbon Nanotubes on MUC5AC and MUC5B Expression in Airway Epithelial Cells
- In Vivo Effects of Adipose-Derived Stem Cells in Inducing Neuronal Regeneration in Sprague-Dawley Rats Undergoing Nerve Defect Bridged with Polycaprolactone Nanotubes