Allergy Asthma Immunol Res.
2015 Jan;7(1):37-43. 10.4168/aair.2015.7.1.37.
Significance of 40-, 45-, and 48-kDa Proteins in the Moderate-to-Severe Clinical Symptoms of Buckwheat Allergy
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Environmental Health Center for Atopic Diseases, Samsung Medical Center, Seoul, Korea. snuhan@skku.edu
- 3Samsung Biomedical Research Institute, Seoul, Korea.
- 4Korea Food Research Institute, Seongnam, Korea.
- 5Korea University of Science and Technology, Daejeon, Korea.
- KMID: 2260150
- DOI: http://doi.org/10.4168/aair.2015.7.1.37
Abstract
- PURPOSE
This study was aimed to investigate the relationship between the allergen components and moderate-to-severe allergic reactions in patients with buckwheat allergy.
METHODS
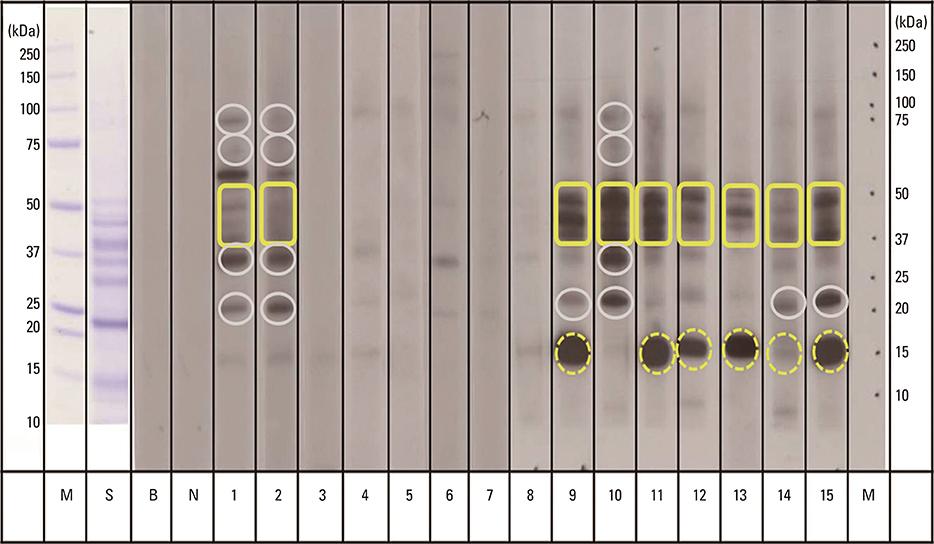
Fifteen patients with a history of buckwheat ingestion and a buckwheat specific IgE level> or =0.35 kU/L were enrolled. They were divided into 2 groups according to clinical severity scores, with 0-1 being asymptomatic-to-mild and 2-4 being moderate-to-severe symptoms. Immunoblotting was performed to investigate IgE reactivity toward buckwheat allergens and to measure intensity of each component by using a reflective densitometer.
RESULTS
The proportions of positive band to the 16 kDa (62.5% vs 0%, P=0.026) and 40-50 kDa (87.5% vs 28.6%, P=0.041) buckwheat allergens in the grade 2-4 group were higher than those in grade 0-1 group. The level of buckwheat specific IgE of grade 2-4 group was higher than that of grade 0-1 group (41.3 kU/L vs 5.5 kU/L, P=0.037). The median optical densities (ODs) of IgE antibody binding to 40-50 kDa protein were higher in the grade 2-4 group, compared with those in the grade 0-1 group (130% OD vs 60.8% OD, P=0.037).
CONCLUSIONS
The 40-50 kDa protein is implicated as an important allergen to predict moderate-to-severe clinical symptoms in Korean children with buckwheat allergy.
Keyword
MeSH Terms
Figure
Reference
-
1. Ahn K, Kim J, Hahm MI, Lee SY, Kim WK, Chae Y, Park YM, Han MY, Lee KJ, Kim JK, Yang ES, Kwon HJ. Prevalence of immediate-type food allergy in Korean schoolchildren: a population-based study. Allergy Asthma Proc. 2012; 33:481–487.2. Lee SY, Lee KS, Hong CH, Lee KY. Three cases of childhood nocturnal asthma due to buckwheat allergy. Allergy. 2001; 56:763–766.3. Lee SY. IgE mediated food allergy in Korean children: focused on plant food allergy. Asia Pac Allergy. 2013; 3:15–22.4. Oh JW, Pyun BY, Choung JT, Ahn KM, Kim CH, Song SW, Son JA, Lee SY, Lee SI. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J Korean Med Sci. 2004; 19:716–723.5. Sohn MH, Lee SY, Kim KE. Prediction of buckwheat allergy using specific IgE concentrations in children. Allergy. 2003; 58:1308–1310.6. Choi SY, Sohn JH, Lee YW, Lee EK, Hong CS, Park JW. Characterization of buckwheat 19-kD allergen and its application for diagnosing clinical reactivity. Int Arch Allergy Immunol. 2007; 144:267–274.7. Choi SY, Sohn JH, Lee YW, Lee EK, Hong CS, Park JW. Application of the 16-kDa buckwheat 2 S storage albumin protein for diagnosis of clinical reactivity. Ann Allergy Asthma Immunol. 2007; 99:254–260.8. Tohgi K, Kohno K, Takahashi H, Matsuo H, Nakayama S, Morita E. Usability of Fag e 2 ImmunoCAP in the diagnosis of buckwheat allergy. Arch Dermatol Res. 2011; 303:635–642.9. Wang Z, Zhang Z, Zhao Z, Wieslander G, Norbäck D, Kreft I. Purification and characterization of a 24 kDa protein from tartary buckwheat seeds. Biosci Biotechnol Biochem. 2004; 68:1409–1413.10. Yoshioka H, Ohmoto T, Urisu A, Mine Y, Adachi T. Expression and epitope analysis of the major allergenic protein Fag e 1 from buckwheat. J Plant Physiol. 2004; 161:761–767.11. Tanaka K, Matsumoto K, Akasawa A, Nakajima T, Nagasu T, Iikura Y, Saito H. Pepsin-resistant 16-kD buckwheat protein is associated with immediate hypersensitivity reaction in patients with buckwheat allergy. Int Arch Allergy Immunol. 2002; 129:49–56.12. Yoshimasu MA, Zhang JW, Hayakawa S, Mine Y. Electrophoretic and immunochemical characterization of allergenic proteins in buckwheat. Int Arch Allergy Immunol. 2000; 123:130–136.13. Lee S, Han Y, Do JR, Oh S. Allergenic potential and enzymatic resistance of buckwheat. Nutr Res Pract. 2013; 7:3–8.14. Matsumoto R, Fujino K, Nagata Y, Hashiguchi S, Ito Y, Aihara Y, Takahashi Y, Maeda K, Sugimura K. Molecular characterization of a 10-kDa buckwheat molecule reactive to allergic patients' IgE. Allergy. 2004; 59:533–538.15. Heffler E, Nebiolo F, Asero R, Guida G, Badiu I, Pizzimenti S, Marchese C, Amato S, Mistrello G, Canaletti F, Rolla G. Clinical manifestations, co-sensitizations, and immunoblotting profiles of buckwheat-allergic patients. Allergy. 2011; 66:264–270.16. Astier C, Morisset M, Roitel O, Codreanu F, Jacquenet S, Franck P, Ogier V, Petit N, Proust B, Moneret-Vautrin DA, Burks AW, Bihain B, Sampson HA, Kanny G. Predictive value of skin prick tests using recombinant allergens for diagnosis of peanut allergy. J Allergy Clin Immunol. 2006; 118:250–256.17. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227:680–685.18. Nakamura S, Yamaguchi M, Oishi M, Hayama T. Studies on the buckwheat allergose report 1: on the cases with the buckwheat allergose. Allerg Immunol (Leipz). 1974; 20-21:449–456.19. Takahashi Y, Ichikawa S, Aihara Y, Yokota S. Buckwheat allergy in 90,000 school children in Yokohama. Arerugi. 1998; 47:26–33.20. Shaker M, Woodmansee D. An update on food allergy. Curr Opin Pediatr. 2009; 21:667–674.21. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001; 107:891–896.22. Sicherer SH, Wood RA. American Academy of Pediatrics Section On Allergy And Immunology. Allergy testing in childhood: using allergen-specific IgE tests. Pediatrics. 2012; 129:193–197.23. Söderström L, Kober A, Ahlstedt S, de Groot H, Lange CE, Paganelli R, Roovers MH, Sastre J. A further evaluation of the clinical use of specific IgE antibody testing in allergic diseases. Allergy. 2003; 58:921–928.24. Nagata Y, Fujino K, Hashiguchi S, Abe N, Zaima Y, Ito Y, Takahashi Y, Maeda K, Sugimura K. Molecular characterization of buckwheat major immunoglobulin E-reactive proteins in allergic patients. Allergol Int. 2000; 49:117–124.25. Park JW, Kang DB, Kim CW, Ko SH, Yum HY, Kim KE, Hong CS, Lee KY. Identification and characterization of the major allergens of buckwheat. Allergy. 2000; 55:1035–1041.26. Kim J, Lee J, Park MR, Han Y, Shin M, Ahn K. Special consideration is required for the component-resolved diagnosis of egg allergy in infants. Ann Allergy Asthma Immunol. 2014; 112:53–57.27. Kim J, Lee JY, Han Y, Ahn K. Significance of Ara h 2 in clinical reactivity and effect of cooking methods on allergenicity. Ann Allergy Asthma Immunol. 2013; 110:34–38.28. Eller E, Bindslev-Jensen C. Clinical value of component-resolved diagnostics in peanut-allergic patients. Allergy. 2013; 68:190–194.29. Bublin M, Pfister M, Radauer C, Oberhuber C, Bulley S, Dewitt AM, Lidholm J, Reese G, Vieths S, Breiteneder H, Hoffmann-Sommergruber K, Ballmer-Weber BK. Component-resolved diagnosis of kiwifruit allergy with purified natural and recombinant kiwifruit allergens. J Allergy Clin Immunol. 2010; 125:687–694. 694.e130. Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, Härlin A, Woodcock A, Ahlstedt S, Custovic A. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010; 125:191–197.e1-13.31. Mittag D, Vieths S, Vogel L, Becker WM, Rihs HP, Helbling A, Wüthrich B, Ballmer-Weber BK. Soybean allergy in patients allergic to birch pollen: clinical investigation and molecular characterization of allergens. J Allergy Clin Immunol. 2004; 113:148–154.32. Ballmer-Weber BK, Wangorsch A, Bohle B, Kaul S, Kündig T, Fötisch K, van Ree R, Vieths S. Component-resolved in vitro diagnosis in carrot allergy: does the use of recombinant carrot allergens improve the reliability of the diagnostic procedure? Clin Exp Allergy. 2005; 35:970–978.