Korean J Hematol.
2008 Sep;43(3):159-165. 10.5045/kjh.2008.43.3.159.
Complications of a Totally Implanted Vascular Access Device (Chemoport) in Children with Malignancy
- Affiliations
-
- 1Department of Pediatrics, Kyungpook National University Hospital, Daegu, Korea. kslee@knu.ac.kr
- KMID: 2252182
- DOI: http://doi.org/10.5045/kjh.2008.43.3.159
Abstract
-
BACKGROUND: Carefully using a totally implanted vascular access device and regular check-up of its condition in children who suffer with malignancy is very important. This study was performed to determine the complications related to using this device, according to the patient's age, gender and diagnosis, and the time from port insertion.
METHODS
We retrospectively studied 77 patients with malignancy (46 males and 31 females, age: 0.1~18 years, mean age: 7.8 years) and they were treated with a totally implanted vascular access device (chemoport) from January 1996 to May 2007 in Kyungpook National University Hospital, Korea. We assessed the symptoms and radiologic findings, conducted blood tests and doppler USG; we found several complications and compared them according the patients' age, gender and diagnosis.
RESULTS
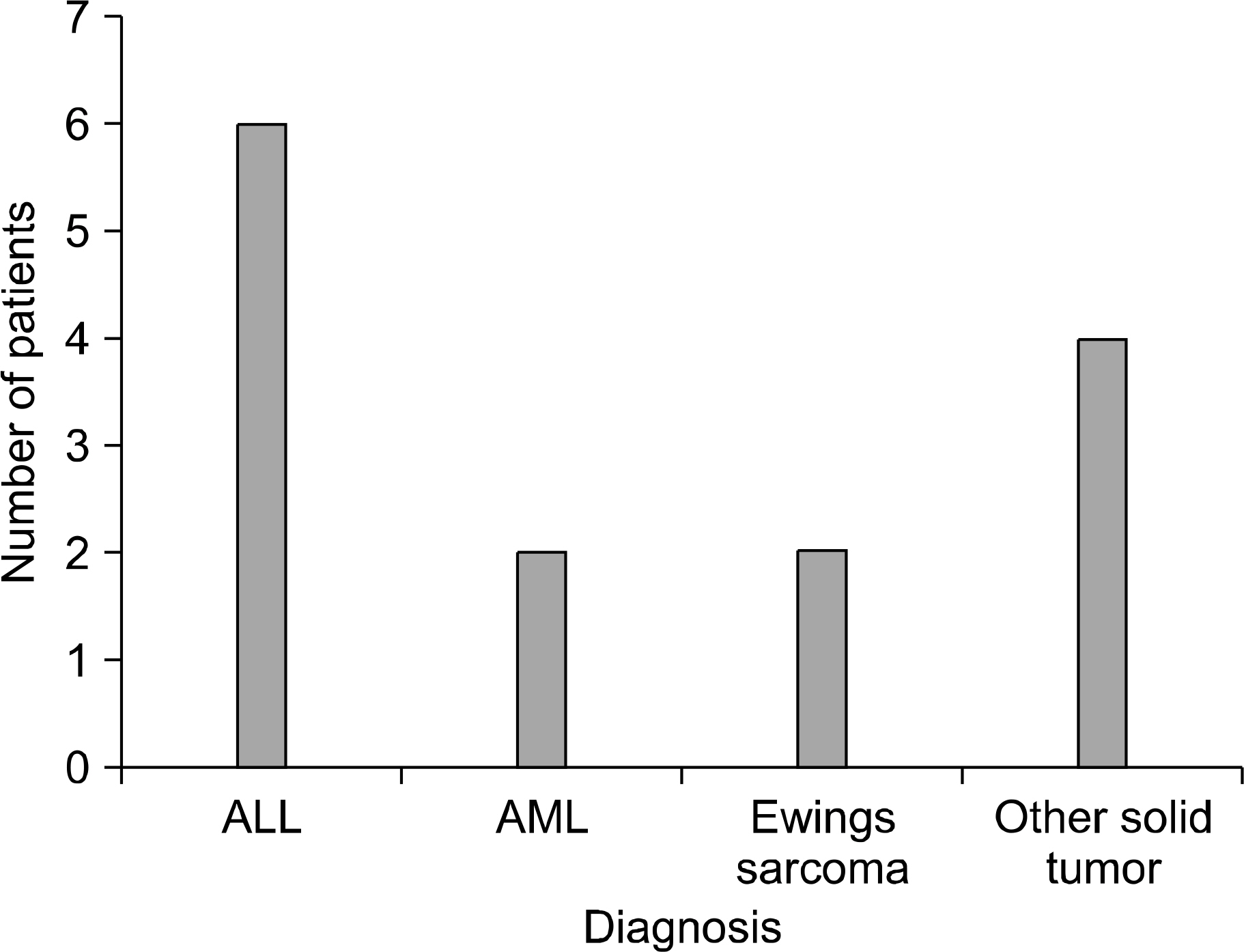
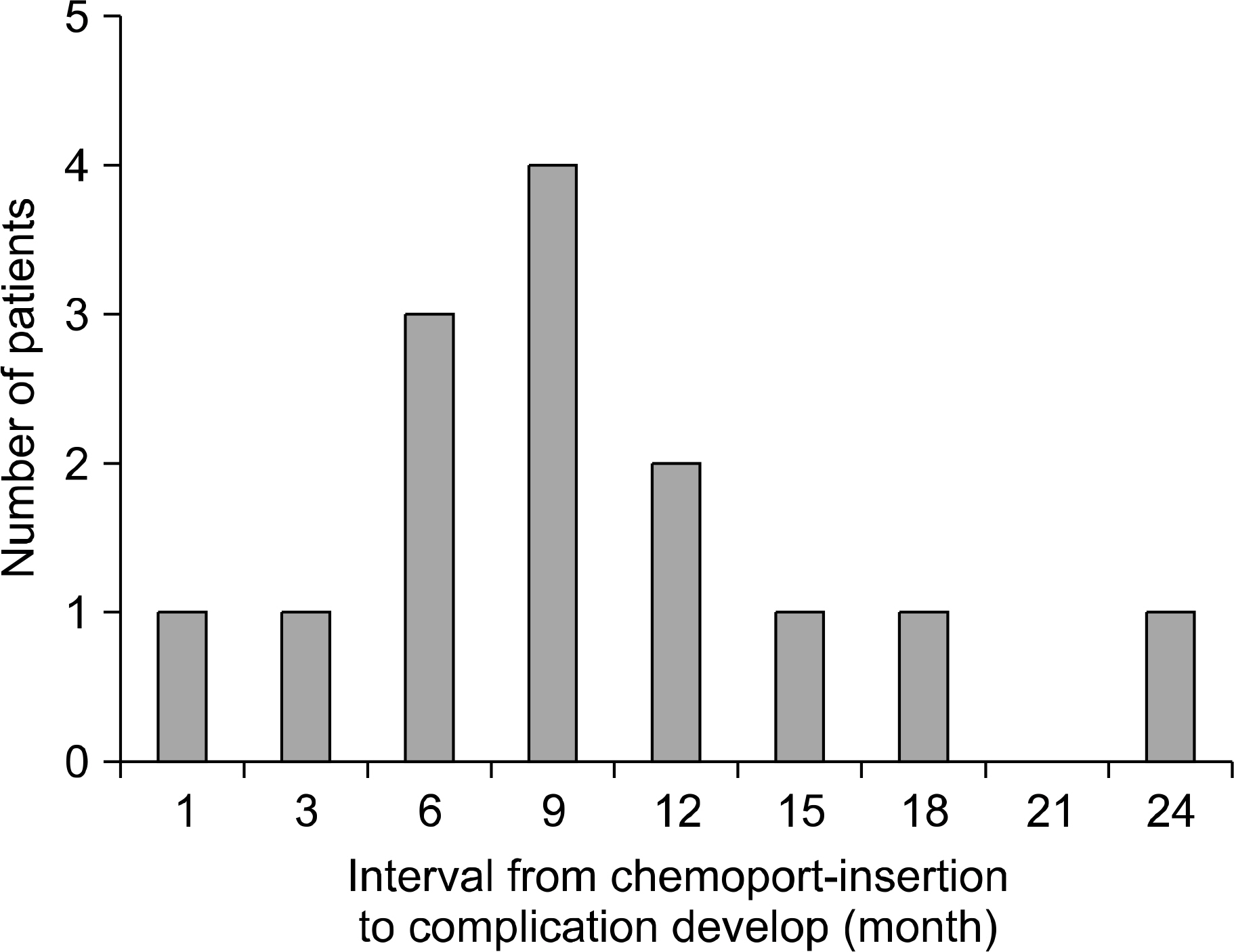
Among the 77 cases with a totally implanted vascular access device (chemoport), 14 cases had complications related to the chemoport. Infections were detected in 8 cases. 6 of them had infections related to the chemoport after 4~7 months from the port-insertion. After port removal and treatment with broad spectrum antibiotics, their symptoms such as fever and swelling were improved. Disconnection of the port was detected in 2 cases after 2 months and 22 months from port-insertion, respectively. These ports were successfully removed by cardiac catheterization. Rotation of the port was detected in one case after 9 months from port-insertion: the rotated port was removed. Obstruction with thrombus was detected in 3 cases, after 7~16 months from port-insertion: this condition was treated with thrombolytic agents such urokinase and t-PA (tissue plasminogen activator), or surgical removal of the blood clot in the port site.
CONCLUSION
To reduce the complications related to the totally implanted vascular access (device), such as infection, thrombosis and disconnection, we should carefully use this device and also regularly check its function and position. After completion of chemotherapy, removal of the port as soon as possible should be considered. If a complication is detected, then we should manage it immediately.
Keyword
MeSH Terms
-
Anti-Bacterial Agents
Cardiac Catheterization
Cardiac Catheters
Child
Female
Fever
Fibrinolytic Agents
Hematologic Tests
Humans
Korea
Male
Plasminogen
Retrospective Studies
Thrombosis
Urokinase-Type Plasminogen Activator
Vascular Access Devices
Anti-Bacterial Agents
Fibrinolytic Agents
Plasminogen
Urokinase-Type Plasminogen Activator
Figure
Reference
-
1). Niederhuber JE., Ensminger W., Gyves JW. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 1982. 92:706–12.2). Dooley WC., Niederhuber JE. Establishing and maintaining vascular access. Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, editors. Clinical oncology. 2nd ed.Churchill Livingstone;2000. p. 755–75.3). Lee YH. Clinical study of Hickman catheters in pediatric oncologic patients. J Korean Pediatr Soc. 1996. 39:682–90.4). Park KD., Dong ES., Ha SH, et al. A prospective study of totally implanted venous access system in 19 children with cancer. J Korean Pediatric Soc. 1993. 36:687–92.5). Choi SH., Cho SH., Kim CJ., Kook H., Hwang TJ. Subclavian catheterization in the pediatric patients. J Korean Pediatr Soc. 1997. 40:368–74.6). Lokich JJ., Bothe A Jr., Benotti P., Moore C. Complications and management of implanted venous access catheters. J Clin Oncol. 1985. 3:710–7.
Article7). Hinke DH., Zandt-Stastny DA., Goodman LR., Queb-beman EJ., Krzywda EA., Andris DA. Pinch-off syndrome: a complication of implanted subclavian venous access devices. Radiology. 1990. 177:353–6.8). Lenglinger FX., Hartl P., Kirchgatterer A., Lenglinger GM., Baldinger C. Fracture and embolization of a central venous port catheter without prior compression between the clavicle and the 1st rib. Wien Klin Wochenschr. 2001. 113:134–7.9). Wu JR., Hsu JH., Chang TT., Dai ZK., Lu CC., Wu DK. Nonsurgical percutaneous retrieval of dislodged Port-A catheters from pulmonary artery in children. Jpn Heart J. 2002. 43:295–300.
Article10). Neuling KF., Peet AC., Stevens MC., Donnell SC. Embolisation of an implanted venous catheter. Med Pediatr Oncol. 2003. 40:409–10.
Article11). Behrend M., Paboura E., Raab R. Late embolization of an unfractured port catheter into the heart: report of a case. Surg Today. 2002. 32:724–6.
Article12). Röggla G., Linkesch M., Röggla M., Wagner A., Haber P., Linkesch W. A rare complication of a central venous catheter system (Port-a-Cath). A case report of a catheter embolization after catheter fracture during power training. Int J Sports Med. 1993. 14:345–6.13). Schwarz RE., Coit DG., Groeger JS. Transcutaneously tunneled central venous lines in cancer patients: an analysis of device-related morbidity factors based on prospective data collection. Ann Surg Oncol. 2000. 7:441–9.
Article14). Balestreri L., De Cicco M., Matovic M., Coran F., Morassult S. Central venous catheter-related thrombosis in clinically asymptomatic oncologic patients: a phlebographic study. Eur J Radiol. 1995. 20:108–11.
Article15). Ray S., Stacey R., Imrie M., Frishie J. A review of 560 Hickman catheter insertions. Anaesthesia. 19956. 51:951–5.
Article16). McGee DC., Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003. 348:1123–33.
Article17). Rosovsky RP., Kuter DJ. Catheter-related thrombosis in cancer patients: pathophysiology, diagnosis, and management. Hematol Oncol Clin North Am. 2005. 19:183–202.
Article18). Lawson M., Bottino JC., Hurtibise MR. The use of urokinase to restore patency of occluded central venous catheters. Am J Intraven Ther Clin Nutr. 1982. 9:29–32.19). Polderman KH., Girbes AR. Central venous catheter use. Part 2: infectious complications. Intensive Care Med. 2002. 28:18–28.20). Gupta H., Araki Y., Davidoff AM, et al. Evaluation of pediatric oncology patients with previous multiple central catheters for vascular access: is Doppler ultrasound needed? Pediatr Blood Cancer. 2007. 48:527–31.
Article21). Craft PS., May J., Dorigo A., Hoy C., Plant A. Hickman catheters: left-sided insertion, male gender, and obesity are associated with an increased risk of complications. Aust NZJ Med. 1996. 26:33–9.
Article22). Randolph AG. Identification of central venous catheter-related infections in infants and children. Pediatr Crit Care Med. 2005. 6(3 Suppl):S19–24.
Article23). De Cicco M., Matovic M., Balestreri L, et al. Central venous thrombosis: an early and frequent complication in cancer patients bearing long-term silastic catheter. A prospective study. Thromb Res. 1997. 86:101–13.
Article24). Eastman ME., Khorsand M., Maki DG, et al. Central venous device-related infection and thrombosis in patients treated with moderate dose continuous-infusion interleukin-2. Cancer. 2001. 91:806–14.
Article25). Glaser DW., Medeiros D., Rollins N., Buchanan GR. Catheter-related thrombosis in children with cancer. J Pediatr. 2001. 138:255–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Disconnection and Migration of Totally Implanted Vascular Access Devices in Three Pediatric Oncology Patients
- Chemoport-A Savior in Children Who Require Chronic Venous Access: An Observational Study
- Successful Removal of a Migrated Catheter of Chemoport in Right Atrium
- A prospective study of totally implanted venous access system in 19 children with cancer
- Catheter Fracture of a Totally Implantable Venous Device Due to Pinch Off Syndrome in Breast Cancer: A Case Report