Korean J Hematol.
2010 Mar;45(1):58-61. 10.5045/kjh.2010.45.1.58.
Efficacy and safety of deferiprone (Ferriprox), an oral iron-chelating agent, in pediatric patients
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Yonsei University, Seoul, Korea. cj@yuhs.ac
- 2Department of Pediatrics, College of Medicine, Chonnam National University Medical School, Hwasun, Korea.
- 3Department of Pediatrics, College of Medicine, Asan Medical Center, University of Ulsan, Seoul, Korea.
- 4Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 5Department of Pediatrics, College of Medicine, University of Ulsan, Ulsan, Korea.
- 6Department of Pediatrics, College of Medicine, Soonchunhyang University, Cheonan, Korea.
- KMID: 2252088
- DOI: http://doi.org/10.5045/kjh.2010.45.1.58
Abstract
- BACKGROUND
Iron overload is a predictable and life-threatening complication in patients dependent on the regular transfusion of RBCs. The aims of this study were to investigate the efficacy and safety of deferiprone in a variety of pediatric hematologic and/or oncologic patients with a high iron overload.
METHODS
Seventeen patients (age: 1.1-20.4 years; median: 10.6 years) from 7 hospitals who were treated with deferiprone from 2006 to 2009 were enrolled in this study. Medical records of enrolled patients were reviewed retrospectively.
RESULTS
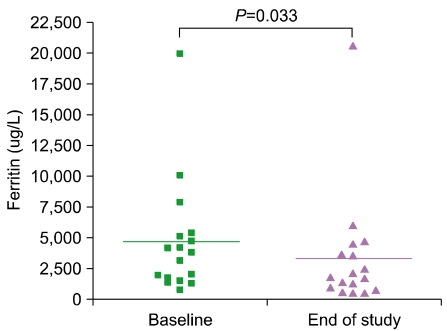
Serum ferritin levels were 4,677.8+/-1,130.9 microgram/L at baseline compared to 3,363.9+/-1,149.7 microgram/L at the end of deferiprone treatment (P=0.033). Only 1 patient developed neutropenia as a complication.
CONCLUSION
Deferiprone treatment is relatively safe for pediatric patients suffering from various hematologic and oncologic diseases that require RBC transfusions as part of treatment. However, the potential development of critical complications such as agranulocytosis and/or neutropenia remains a concern.
Keyword
MeSH Terms
Figure
Reference
-
1. Gabutti V, Piga A. Results of long-term iron-chelating therapy. Acta Haematol. 1996; 95:26–36. PMID: 8604584.
Article2. Zurlo MG, De Stefano P, Borgna-Pignatti C, et al. Survival and causes of death in thalassaemia major. Lancet. 1989; 2:27–30. PMID: 2567801.
Article3. Olivieri NF, Nathan DG, MacMillan JH, et al. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med. 1994; 331:574–578. PMID: 8047081.4. Sonakul D, Thakerngpol K, Pacharee P. Cardiac pathology in 76 thalassemic patients. Birth Defects Orig Artic Ser. 1988; 23:177–191. PMID: 3390539.5. Barman Balfour JA, Foster RH. Deferiprone: a review of its clinical potential in iron overload in beta-thalassaemia major and other transfusion-dependent diseases. Drugs. 1999; 58:553–578. PMID: 10493280.6. Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997; 89:739–761. PMID: 9028304.
Article7. Brittenham GM, Griffith PM, Nienhuis AW, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994; 331:567–573. PMID: 8047080.
Article8. Olivieri NF. Long-term therapy with deferiprone. Acta Haematol. 1996; 95:37–48. PMID: 8604585.
Article9. al-Refaie FN, Wonke B, Hoffbrand AV, Wickens DG, Nortey P, Kontoghiorghes GJ. Efficacy and possible adverse effects of the oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one (L1) in thalassemia major. Blood. 1992; 80:593–599. PMID: 1638018.10. Porter J. Oral iron chelators: prospects for future development. Eur J Haematol. 1989; 43:271–285. PMID: 2684681.
Article11. Olivieri NF, Buncic JR, Chew E, et al. Visual and auditory neurotoxicity in patients receiving subcutaneous deferoxamine infusions. N Engl J Med. 1986; 314:869–873. PMID: 3485251.
Article12. Taher A, Abou-Mourad Y, Abchee A, Zalouaa P, Shamseddine A. Pulmonary thromboembolism in beta-thalassemia intermedia: are we aware of this complication? Hemoglobin. 2002; 26:107–112. PMID: 12144052.13. Maggio A, D'Amico G, Morabito A, et al. Deferiprone versus deferoxamine in patients with thalassemia major: a randomized clinical trial. Blood Cells Mol Dis. 2002; 28:196–208. PMID: 12064916.
Article14. Piga A, Gaglioti C, Fogliacco E, Tricta F. Comparative effects of deferiprone and deferoxamine on survival and cardiac disease in patients with thalassemia major: a retrospective analysis. Haematologica. 2003; 88:489–496. PMID: 12745268.15. Fischer R, Longo F, Nielsen P, Engelhardt R, Hider RC, Piga A. Monitoring long-term efficacy of iron chelation therapy by deferiprone and desferrioxamine in patients with beta-thalassaemia major: application of SQUID biomagnetic liver susceptometry. Br J Haematol. 2003; 121:938–948. PMID: 12786807.16. Peng CT, Chow KC, Chen JH, Chiang YP, Lin TY, Tsai CH. Safety monitoring of cardiac and hepatic systems in beta-thalassemia patients with chelating treatment in Taiwan. Eur J Haematol. 2003; 70:392–397. PMID: 12756022.17. Mazza P, Amurri B, Lazzari G, et al. Oral iron chelating therapy. A single center interim report on deferiprone (L1) in thalassemia. Haematologica. 1998; 83:496–501. PMID: 9676021.18. Olivieri NF, Brittenham GM, Matsui D, et al. Iron-chelation therapy with oral deferipronein patients with thalassemia major. N Engl J Med. 1995; 332:918–922. PMID: 7877649.19. Olivieri NF, Brittenham GM, McLaren CE, et al. Long-term safety and effectiveness of iron-chelation therapy with deferiprone for thalassemia major. N Engl J Med. 1998; 339:417–423. PMID: 9700174.
Article20. Ceci A, Baiardi P, Felisi M, et al. The safety and effectiveness of deferiprone in a large-scale, 3-year study in Italian patients. Br J Haematol. 2002; 118:330–336. PMID: 12100170.
Article21. Modell B, Berdoukas V, editors. Clinical Approach to Thalassaemia. 1984. London: Grune & Stratton.22. Cohen AR, Galanello R, Piga A, Dipalma A, Vullo C, Tricta F. Safety profile of the oral iron chelator deferiprone: a multicentre study. Br J Haematol. 2000; 108:305–312. PMID: 10691860.
Article23. Orkin SH, Nathan DG, editors. The Thalassemias. 1998. Philadelphia: W.B. Saunders Company.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deferiprone Related Arthropathy in 11-Year-Old Boy with Hereditary Spherocytosis
- Effect of Iron-Chelator Deferiprone on the In Vitro Growth of Staphylococci
- Efficacy and safety of combined oral iron chelation therapy with deferasirox and deferiprone in a patient with beta-thalassemia major and persistent iron overload
- Economic Evaluation of Iron Chelation Agents: Oral Deferasirox versus Infusional Deferoxamine
- Treatment of Pantothenate-Kinase Neurodegeneration With Baclofen, Botulinum Toxin, and Deferiprone: A Case Report