Korean J Hepatobiliary Pancreat Surg.
2015 May;19(2):47-58. 10.14701/kjhbps.2015.19.2.47.
Toward angiogenesis of implanted bio-artificial liver using scaffolds with type I collagen and adipose tissue-derived stem cells
- Affiliations
-
- 1Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. kskim88@yuhs.ac
- 2Graduate Program of Nano Science and Technology, Graduate School of Yonsei University, Seoul, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 4Cell Therapy Center, Severance Hospital, Seoul, Korea.
- KMID: 2243049
- DOI: http://doi.org/10.14701/kjhbps.2015.19.2.47
Abstract
- BACKGROUNDS/AIMS
Stem cell therapies for liver disease are being studied by many researchers worldwide, but scientific evidence to demonstrate the endocrinologic effects of implanted cells is insufficient, and it is unknown whether implanted cells can function as liver cells. Achieving angiogenesis, arguably the most important characteristic of the liver, is known to be quite difficult, and no practical attempts have been made to achieve this outcome. We carried out this study to observe the possibility of angiogenesis of implanted bio-artificial liver using scaffolds.
METHODS
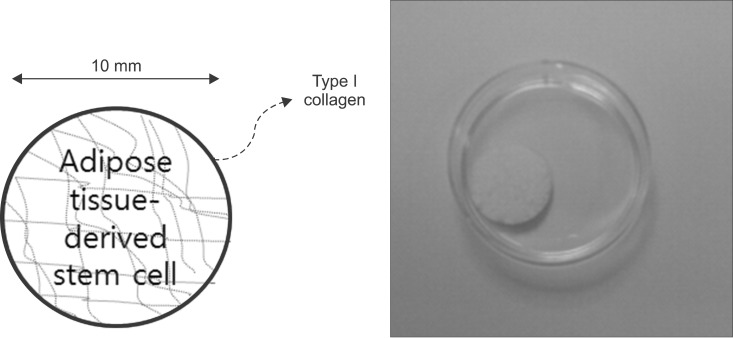
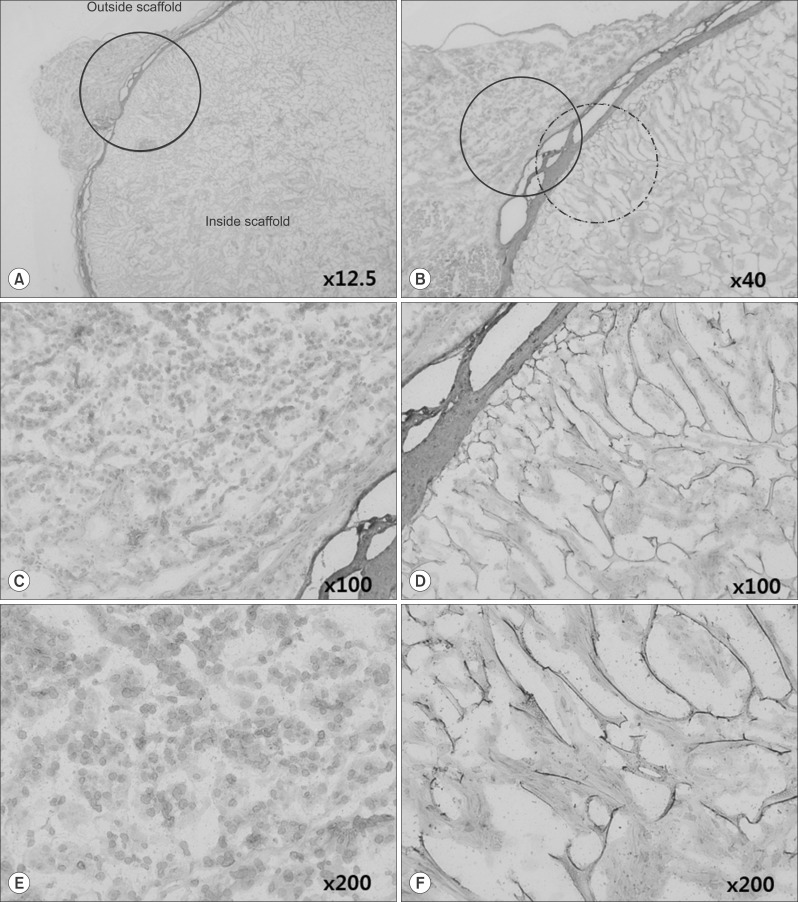
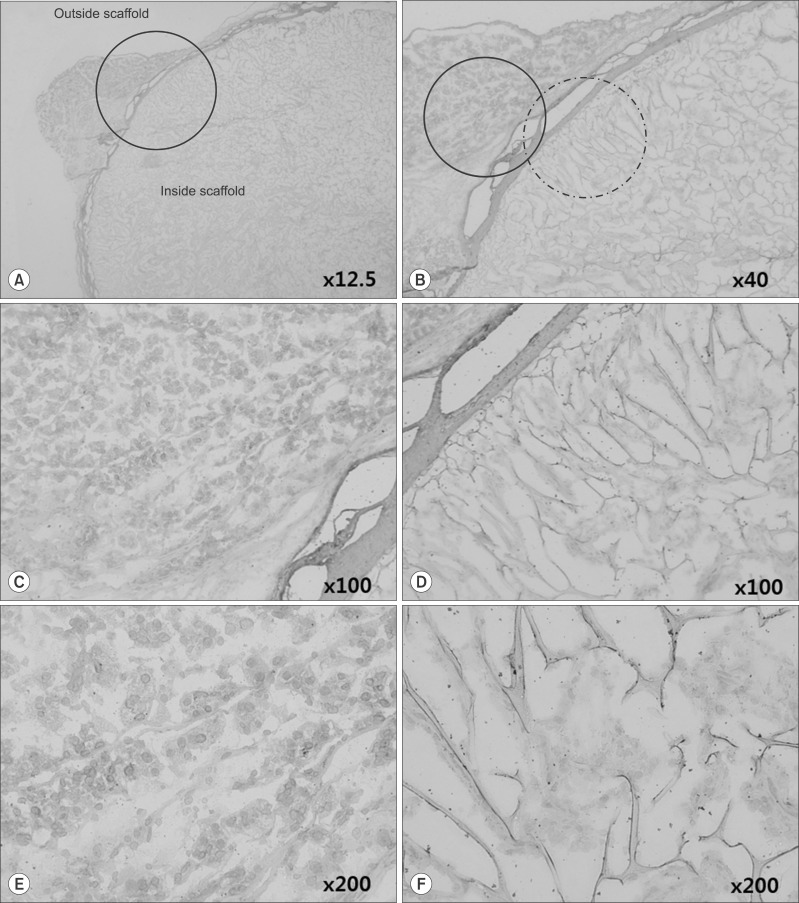
This study used adipose tissue-derived stem cells that were collected from adult patients with liver diseases with conditions similar to the liver parenchyma. Specifically, microfilaments were used to create an artificial membrane and maintain the structure of an artificial organ. After scratching the stomach surface of severe combined immunocompromised (SCID) mice (n=4), artificial scaffolds with adipose tissue-derived stem cells and type I collagen were implanted. Expression levels of angiogenesis markers including vascular endothelial growth factor (VEGF), CD34, and CD105 were immunohistochemically assessed after 30 days.
RESULTS
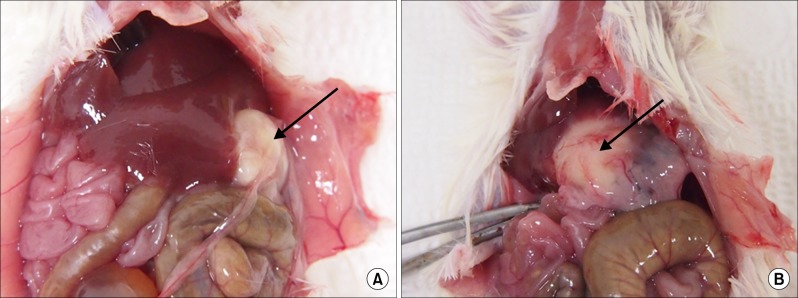
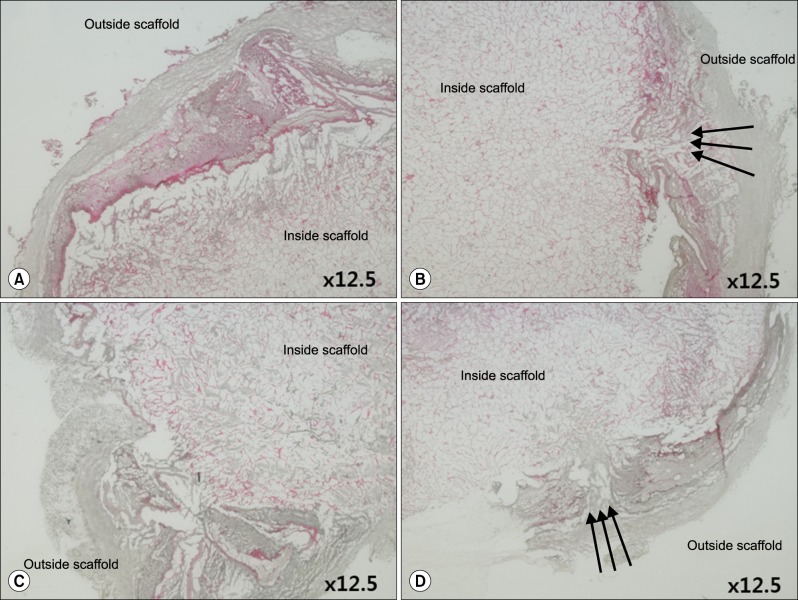
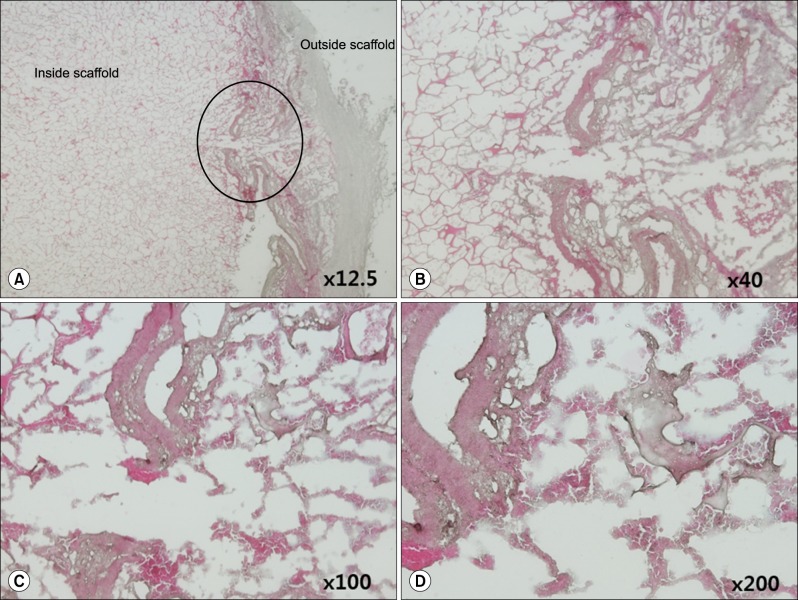
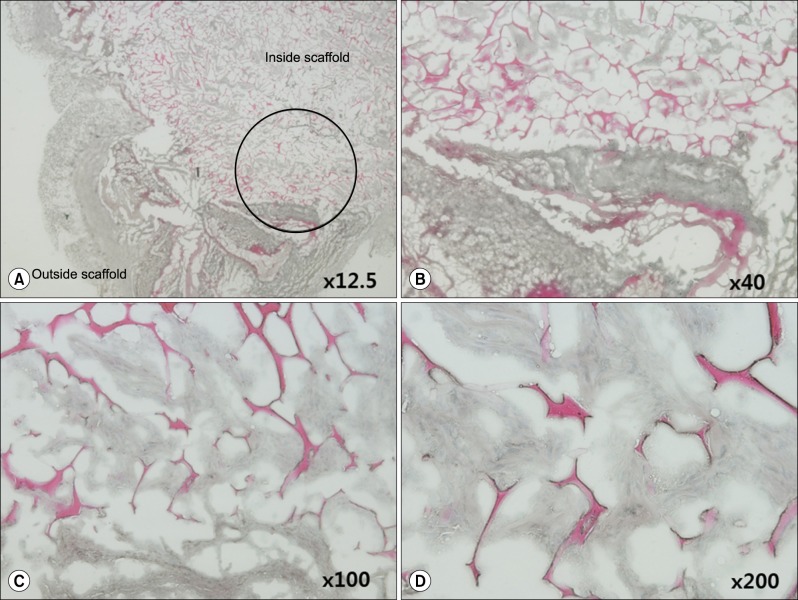
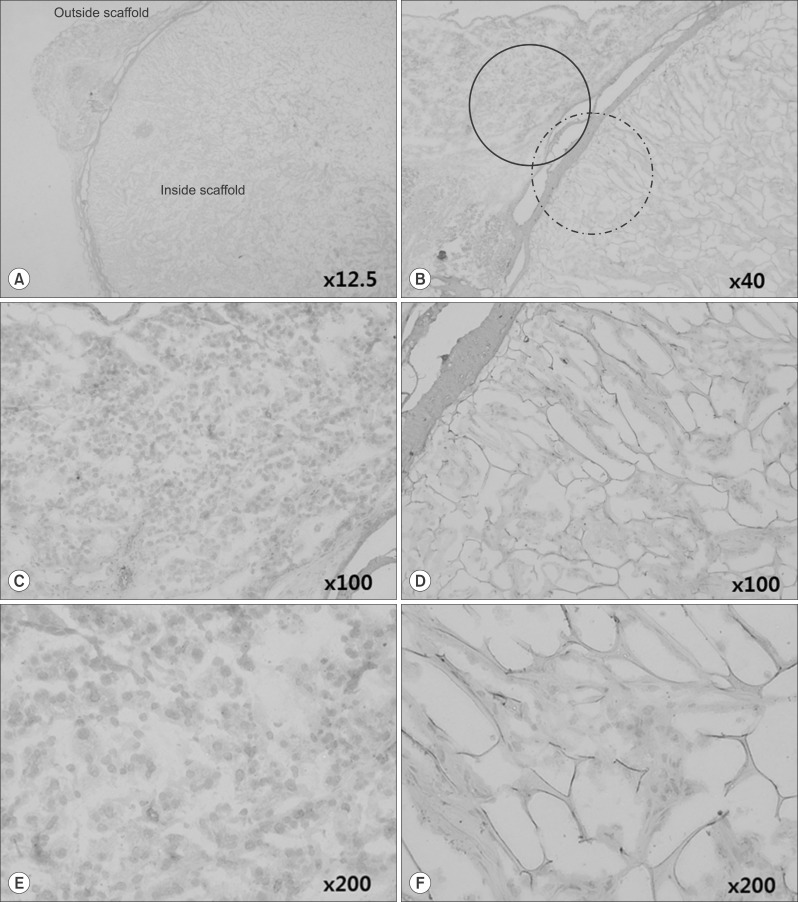
Grossly, the artificial scaffolds showed adhesion to the stomach and surrounding organs; however, there was no evidence of angiogenesis within the scaffolds; and VEGF, CD34, and CD105 expressions were not detected after 30 days.
CONCLUSIONS
Although implantation of cells into artificial scaffolds did not facilitate angiogenesis, the artificial scaffolds made with type I collagen helped maintain implanted cells, and surrounding tissue reactions were rare. Our findings indicate that type I collagen artificial scaffolds can be considered as a possible implantable biomaterial.
Keyword
MeSH Terms
-
Actin Cytoskeleton
Adult
Animals
Artificial Organs
Biocompatible Materials
Collagen Type I*
Humans
Liver Diseases
Liver*
Membranes, Artificial
Mice
Stem Cells*
Stomach
Tissue Scaffolds
Vascular Endothelial Growth Factor A
Biocompatible Materials
Collagen Type I
Membranes, Artificial
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Korean Network for Organ Sharing. Annual Report of the transplant 2013. Ministry of Welfare;2014.2. Dutkowski P, Oberkofler CE, Béchir M, Müllhaupt B, Geier A, Raptis DA, et al. The model for end-stage liver disease allocation system for liver transplantation saves lives, but increases morbidity and cost: a prospective outcome analysis. Liver Transpl. 2011; 17:674–684. PMID: 21618688.
Article3. Axelrod DA. Economic and financial outcomes in transplantation: whose dime is it anyway? Curr Opin Organ Transplant. 2013; 18:222–228. PMID: 23449346.4. Dutkowski P, Oberkofler CE, Slankamenac K, Puhan MA, Schadde E, Müllhaupt B, et al. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. 2011; 254:745–753. PMID: 22042468.5. Struecker B, Raschzok N, Sauer IM. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol. 2014; 11:166–176. PMID: 24166083.
Article6. Iwata H, Ueda Y. Pharmacokinetic considerations in development of a bioartificial liver. Clin Pharmacokinet. 2004; 43:211–225. PMID: 15005636.
Article7. Sauer IM, Kardassis D, Zeillinger K, Pascher A, Gruenwald A, Pless G, et al. Clinical extracorporeal hybrid liver support--phase I study with primary porcine liver cells. Xenotransplantation. 2003; 10:460–469. PMID: 12950989.8. Pascher A, Sauer IM, Neuhaus P. Analysis of allogeneic versus xenogeneic auxiliary organ perfusion in liver failure reveals superior efficacy of human livers. Int J Artif Organs. 2002; 25:1006–1012. PMID: 12456043.
Article9. Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, et al. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998; 153:319–329. PMID: 9665494.
Article10. Dhawan A, Strom SC, Sokal E, Fox IJ. Human hepatocyte transplantation. Methods Mol Biol. 2010; 640:525–534. PMID: 20645072.
Article11. Gupta S, Rajvanshi P, Sokhi R, Slehria S, Yam A, Kerr A, et al. Entry and integration of transplanted hepatocytes in rat liver plates occur by disruption of hepatic sinusoidal endothelium. Hepatology. 1999; 29:509–519. PMID: 9918929.
Article12. Ambrosino G, Varotto S, Strom SC, Guariso G, Franchin E, Miotto D, et al. Isolated hepatocyte transplantation for Crigler-Najjar syndrome type 1. Cell Transplant. 2005; 14:151–157. PMID: 15881424.
Article13. Darwish AA, Sokal E, Stephenne X, Najimi M, de Goyet Jde V, Reding R. Permanent access to the portal system for cellular transplantation using an implantable port device. Liver Transpl. 2004; 10:1213–1215. PMID: 15350017.
Article14. Dhawan A, Mitry RR, Hughes RD. Hepatocyte transplantation for liver-based metabolic disorders. J Inherit Metab Dis. 2006; 29:431–435. PMID: 16763914.
Article15. Allen KJ, Mifsud NA, Williamson R, Bertolino P, Hardikar W. Cell-mediated rejection results in allograft loss after liver cell transplantation. Liver Transpl. 2008; 14:688–694. PMID: 18433045.
Article16. Lysy PA, Najimi M, Stephenne X, Bourgois A, Smets F, Sokal EM. Liver cell transplantation for Crigler-Najjar syndrome type I: update and perspectives. World J Gastroenterol. 2008; 14:3464–3470. PMID: 18567072.
Article17. Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011; 480:547–551. PMID: 22056989.
Article18. Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008; 26:443–452. PMID: 18288110.
Article19. Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010; 51:297–305. PMID: 19998274.
Article20. Han HS, Ahn KS, Cho JY, Yoon YS, Yoon CJ, Park KU, et al. Autologous stem cell transplantation for expansion of remnant liver volume with extensive hepatectomy. Hepatogastroenterology. 2014; 61:156–161. PMID: 24895813.21. Moon JJ, West JL. Vascularization of engineered tissues: approaches to promote angio-genesis in biomaterials. Curr Top Med Chem. 2008; 8:300–310. PMID: 18393893.22. Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011; 17:1359–1370. PMID: 22064426.
Article23. Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014; 123:625–631. PMID: 24300855.
Article24. Crafts TD, Jensen AR, Blocher-Smith EC, Markel TA. Vascular endothelial growth factor: therapeutic possibilities and challenges for the treatment of ischemia. Cytokine. 2015; 71:385–393. PMID: 25240960.
Article25. West CM, Cooper RA, Loncaster JA, Wilks DP, Bromley M. Tumor vascularity: a histological measure of angiogenesis and hypoxia. Cancer Res. 2001; 61:2907–2910. PMID: 11306466.26. Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. Vascularization--the conduit to viable engineered tissues. Tissue Eng Part B Rev. 2009; 15:159–169. PMID: 19309238.
Article27. Li LH, Guo ZJ, Yan LL, Yang JC, Xie YF, Sheng WH, et al. Antitumor and antiangiogenic activities of anti-vascular endothelial growth factor hairpin ribozyme in human hepatocellular carcinoma cell cultures and xenografts. World J Gastroenterol. 2007; 13:6425–6432. PMID: 18081234.
Article28. Ng YS, Krilleke D, Shima DT. VEGF function in vascular pathogenesis. Exp Cell Res. 2006; 312:527–537. PMID: 16330026.
Article29. Maharaj AS, D'Amore PA. Roles for VEGF in the adult. Microvasc Res. 2007; 74:100–113. PMID: 17532010.
Article30. Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007; 19:2003–2012. PMID: 17658244.
Article31. Pisacane AM, Picciotto F, Risio M. CD31 and CD34 expression as immunohistochemical markers of endothelial transdifferentiation in human cutaneous melanoma. Cell Oncol. 2007; 29:59–66. PMID: 17429142.
Article32. Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006; 54:385–395. PMID: 16234507.
Article33. Westra WH, Gerald WL, Rosai J. Solitary fibrous tumor. Consistent CD34 immunoreactivity and occurrence in the orbit. Am J Surg Pathol. 1994; 18:992–998. PMID: 7522416.
Article34. Kutzner H. Expression of the human progenitor cell antigen CD34 (HPCA-1) distinguishes dermatofibrosarcoma protuberans from fibrous histiocytoma in formalin-fixed, paraffin-embedded tissue. J Am Acad Dermatol. 1993; 28:613–617. PMID: 7681857.
Article35. Vasconcelos MG, Alves PM, Vasconcelos RG, da Silveira ÉJ, Medeiros AM, de Queiroz LM. Expression of CD34 and CD105 as markers for angiogenesis in oral vascular malformations and pyogenic granulomas. Eur Arch Otorhinolaryngol. 2011; 268:1213–1217. PMID: 21221622.
Article36. Popa ER, Harmsen MC, Tio RA, van der Strate BW, Brouwer LA, Schipper M, et al. Circulating CD34+ progenitor cells modulate host angiogenesis and inflammation in vivo. J Mol Cell Cardiol. 2006; 41:86–96. PMID: 16780869.
Article37. Vieira SC, Silva BB, Pinto GA, Vassallo J, Moraes NG, Santana JO, et al. CD34 as a marker for evaluating angiogenesis in cervical cancer. Pathol Res Pract. 2005; 201:313–318. PMID: 15991838.
Article38. Kimura H, Nakajima T, Kagawa K, Deguchi T, Kakusui M, Katagishi T, et al. Angiogenesis in hepatocellular carcinoma as evaluated by CD34 immunohistochemistry. Liver. 1998; 18:14–19. PMID: 9548262.
Article39. Karavasilis V, Malamou-Mitsi V, Briasoulis E, Tsanou E, Kitsou E, Kalofonos H, et al. Angiogenesis in cancer of unknown primary: clinicopathological study of CD34, VEGF and TSP-1. BMC Cancer. 2005; 5:25. PMID: 15743540.
Article40. Fonsatti E, Maio M. Highlights on endoglin (CD105): from basic findings towards clinical applications in human cancer. J Transl Med. 2004; 2:18. PMID: 15193152.41. Fonsatti E, Del Vecchio L, Altomonte M, Sigalotti L, Nicotra MR, Coral S, et al. Endoglin: An accessory component of the TGF-beta-binding receptor-complex with diagnostic, prognostic, and bioimmunotherapeutic potential in human malignancies. J Cell Physiol. 2001; 188:1–7. PMID: 11382917.42. Sharma S, Sharma MC, Sarkar C. Morphology of angiogenesis in human cancer: a conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology. 2005; 46:481–489. PMID: 15842629.
Article43. Wang D, Stockard CR, Harkins L, Lott P, Salih C, Yuan K, et al. Immunohistochemistry in the evaluation of neovascularization in tumor xenografts. Biotech Histochem. 2008; 83:179–189. PMID: 18846440.
Article44. Farrell DJ, Bulmer E, Angus B, Ashcroft T. Immunohistochemical expression of endothelial markers in left atrial myxomas: a study of six cases. Histopathology. 1996; 28:147–152. PMID: 8834523.
Article45. Kademani D, Lewis JT, Lamb DH, Rallis DJ, Harrington JR. Angiogenesis and CD34 expression as a predictor of recurrence in oral squamous cell carcinoma. J Oral Maxillofac Surg. 2009; 67:1800–1805. PMID: 19686913.
Article46. van Amerongen MJ, Molema G, Plantinga J, Moorlag H, van Luyn MJ. Neovascularization and vascular markers in a foreign body reaction to subcutaneously implanted degradable biomaterial in mice. Angiogenesis. 2002; 5:173–180. PMID: 12831058.47. Du C, Narayanan K, Leong MF, Wan AC. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials. 2014; 35:6006–6014. PMID: 24780169.
Article48. Zacchi V, Soranzo C, Cortivo R, Radice M, Brun P, Abatangelo G. In vitro engineering of human skin-like tissue. J Biomed Mater Res. 1998; 40:187–194. PMID: 9549613.
Article49. Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001; 7:1035–1040. PMID: 11533707.
Article50. Hakenberg OW. Re: tissue-engineered autologous bladders for patients needing cystoplasty. Eur Urol. 2006; 50:382–383. PMID: 18219730.
Article51. Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002; 295:1009–1014. PMID: 11834815.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Osteogenic Differentiation of Human Adipose-derived Stem Cells within PLGA(Poly(D,L-lactic-co-glycolic acid)) Scaffold in the Nude Mouse
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation
- Biomimetic Polymer Scaffolds to Promote Stem Cell-Mediated Osteogenesis
- Tissue Engineering and Stem Cells
- Immunomodulatory Effects of Adipose Tissue-Derived Stem Cells on Elastin Scaffold Remodeling in Diabetes