Korean Circ J.
2009 Dec;39(12):505-511. 10.4070/kcj.2009.39.12.505.
Recent Insights Into the Mechanisms of Vasospastic Angina
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, University of Ulsan College of Medicine, Gangneung Asan Hospital, Gangneung, Korea. bovio@naver.com
- 2Division of Cardiology, Department of Internal Medicine, Wonju College of Medicine, Yonsei University, Wonju, Korea.
- KMID: 2225637
- DOI: http://doi.org/10.4070/kcj.2009.39.12.505
Abstract
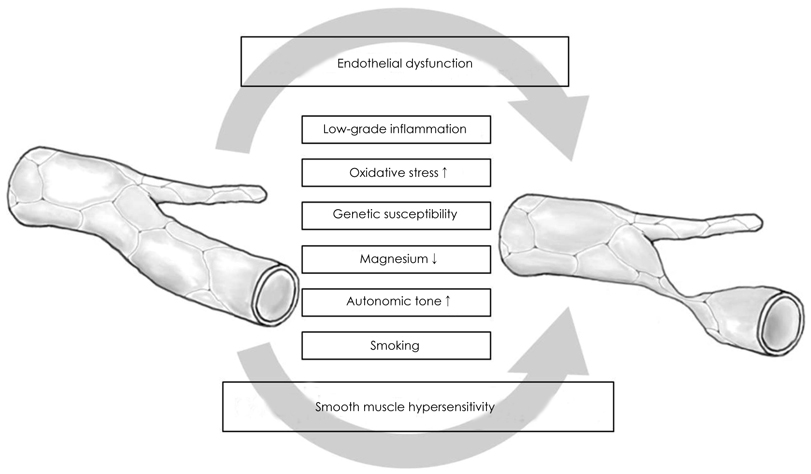
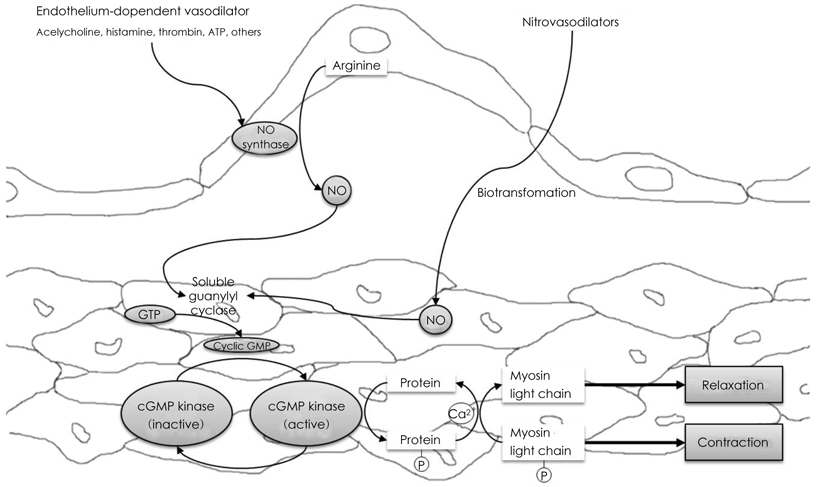
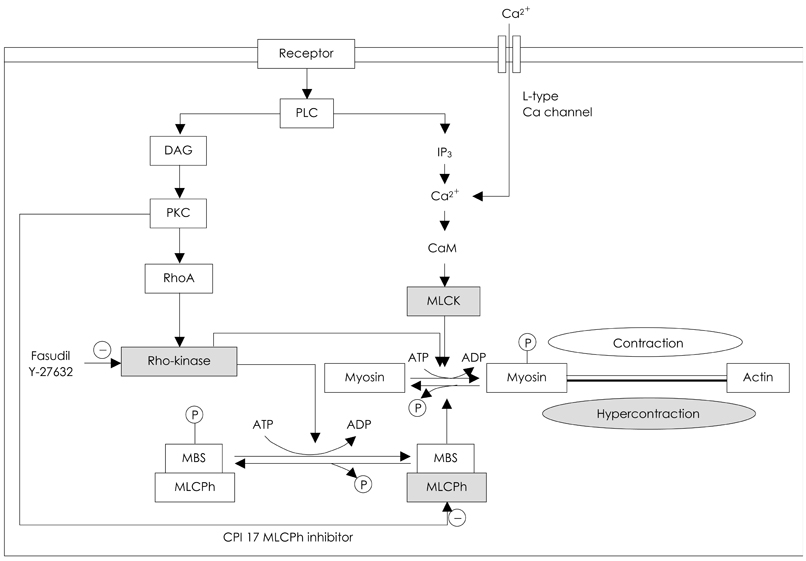
- Coronary artery spasm plays an important role in the pathogenesis of many types of ischemic heart disease, not only in vasospastic angina but also in myocardial infarction and sudden death, particularly in the asian population. Patients with vasospastic angina are known to have defective endothelial function due to reduced nitric oxide bioavailability. Moreover, markers of oxidative stress and plasma levels of C-reactive protein are elevated. Smoking, polymorphysms of endothelial nitric oxide synthetase (eNOS), and low-grade inflammation have been regarded as the most important risk factors for vasospastic angina. The recent body of evidence indicates that RhoA and its down stream effector, ROCK/Rho-kinase, are associated with hypercontraction of vascular smooth muscle of the coronary artery and regulation of eNOS activity. Thus, endothelial dysfunction through abnormalities of eNOS and enhanced contractility of vascular smooth muscle in coronary artery segments are considered major mechanisms in vasospastic angina. However, the precise mechanisms for coronary vasospasm are not well understood. This article will review current understanding of the mechanism of coronary artery spasm.
MeSH Terms
-
Asian Continental Ancestry Group
Biological Availability
C-Reactive Protein
Coronary Vasospasm
Coronary Vessels
Death, Sudden
Endothelium, Vascular
Humans
Inflammation
Muscle, Smooth, Vascular
Myocardial Infarction
Myocardial Ischemia
Nitric Oxide
Nitric Oxide Synthase
Oxidative Stress
Plasma
Risk Factors
Rivers
Smoke
Smoking
Spasm
C-Reactive Protein
Nitric Oxide
Nitric Oxide Synthase
Smoke
Figure
Cited by 1 articles
-
Statins in Variant Angina
Jang-Young Kim
J Cardiovasc Ultrasound. 2013;21(2):56-57. doi: 10.4250/jcu.2013.21.2.56.
Reference
-
1. Sueda S, Kohno H, Fukuda H, Uraoka T. Did the widespread use of long-acting calcium antagonists decrease the occurrence of variant angina? Chest. 2003. 124:2074–2078.2. Yoo SY, Shin DH, Jeong JI, et al. Long-term prognosis and clinical characteristics of patients with variant angina. Korean Circ J. 2008. 38:651–658.3. Curry RC, Pepine CJ, Sabom MB, Feldman RL, Christie LG, Conti CR. Effects of ergonovine in patients with and without coronary artery disease. Circulation. 1977. 56:803–809.4. Maseri A, Davies G, Hackett D, Kaski JC. Coronary artery spasm and vasoconstriction: the case for a distinction. Circulation. 1990. 81:1983–1991.5. Barbara AK, Daniel S, Andrew IS. Libby P, Braunwald E, editors. Hemostasis, thrombosis, fibrinolysis, and cardiovascular disease. Braunwald's Heart disease: A Textbook of Cardiovascular Medicine. 2008. 8th ed. Philadelphia: Saunders company;2049.6. Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002. 105:546–549.7. Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993. 22:Suppl 4. S1–S14.8. Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003. 9:702–712.9. Gulati R, Jevremovic D, Peterson TE, et al. Autologous culture-modified mono-nuclear cells confer vascular protection after arterial injury. Circulation. 2003. 108:1520–1526.10. Aicher A, Heechen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003. 9:1370–1376.11. Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993. 329:2002–2012.12. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991. 43:109–142.13. Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historial overview. J Physiol Pharmacol. 2002. 53:503–514.14. Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003. 348:593–600.15. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005. 111:363–368.16. Vanhoutte PM. Endothelial control of vasomotor function: from health to coronary disease. Circ J. 2003. 67:572–575.17. Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004. 24:998–1005.18. Maseri A, L'Abbate A, Biagini A, et al. Coronary vasospasm as a possible cause of myocardial infarction: a conclusion derived from the study of "preinfarction" angina. N Engl J Med. 1978. 299:1271–1277.19. MacAlpin RN. Angiographic determination of disease-produced alterations of vasomotor tone in large coronary arteries. Can J Cardiol. 1986. Suppl A. 209A–218A.20. Maseri A, Severi S, Nes MD, et al. "Variant" angina: one aspect of a continuous spectrum of vasospastic myocardial ischemia: pathogenic mechanisms, estimated incidence and clinical and coronary arteriographic findings in 138 patients. Am J Cardiol. 1978. 42:1019–1035.21. MacAlpin RN. Correlation of the location of coronary arterial spasm with the lead distribution of ST segment elevation during variant angina. Am Heart J. 1980. 99:555–564.22. Miyao Y, Kugiyama K, Yawano H, et al. Diffuse intimal thickening of coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 2000. 36:432–437.23. Suzuki H, Kawai S, Aizawa T, et al. Histological evaluation of coronary plaques in patients with variant angina: relationship between vasospasm and neointimal hyperplasia in primary coronary lesions. J Am Coll Cardiol. 1999. 33:198–205.24. Shimokawa H, Tomioke H, Nabeyama S, et al. Coronary artery spasm induced in atherosclerotic miniature swine. Science. 1983. 221:560–562.25. Shimokawa H, Tomioke H, Nabeyama S, et al. Coronary artery spasm induced in miniature swine: angiographic evidence and relation to coronary atherosclerosis. Am Heart J. 1985. 110:300–310.26. Miwa K, Fujita M, Sasayama S. Recent insights into the mechanisms, predisposing factors, and racial differences of coronary vasospasm. Heart Vessels. 2005. 20:1–7.27. Suzuki Y, Tokunaga S, Ikegushi S, et al. Induction of coronary artery spasm by intracoronary acetylcholine: comparison with intracoronary ergonovine. Am Heart J. 1992. 124:39–47.28. Kanazawa K, Suematsu M, Ishida T, et al. Disparity between serotonin-and acetylcholine-provoked coronary artery spasm. Clin Cardiol. 1997. 20:146–152.29. Egashira K, Katsuda Y, Mohri M, et al. Basal release of endothelium-derived nitric oxide at site of spasm in patients with variant angina. J Am Coll Cardiol. 1996. 27:1444–1449.30. Yamamoto H, Yoshimura H, Noma M, et al. Preservation of endothelium-dependent vasodilatation in the spastic segment of the human epicardial coronary artery by substance P. Am Heart J. 1992. 123:298–303.31. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980. 288:373–376.32. Okumura K, Yasue H, Horio Y, et al. Multi-vessel coronary spasm in patients with variant angina: a study with intracoronary injection of acetylcholine. Circulation. 1988. 77:535–542.33. Kugiyama K, Yasue H, Okumura K, et al. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation. 1996. 94:266–271.34. Matsuyama K, Yasue H, Okumura K, et al. Effects of H1-receptor stimulation on coronary arterial diameter and coronary hemodynamics in human. Circulation. 1990. 81:65–71.35. Bassenge E. Endothelium-mediated regulation of coronary tone. Basic Res Cardiol. 1991. 86:Suppl 2. 69–76.36. Yoshimura M, Yasue H, Nakayama M, et al. A missense Glu298-Asp variant in the endothelial nitric oxide synthase gene is associated with coronary spasm in the Japanese. Hum Genet. 1998. 103:65–69.37. Nakayama M, Yasue H, Yoshimura M, et al. T786→C mutation in the 5'-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation. 1999. 99:2864–2870.38. Sugiishi M, Takatsu F. Cigarette smoking is a major risk factor for coronary spasm. Circulation. 1993. 87:76–79.39. Murohara T, Kugiyama K, Ohgushi M, Sugiyama S, Yasue H. Cigarette smoke extract contracts isolated porcine coronary arteries by superoxide anion-mediated degradation of EDRF. Am J Physiol. 1994. 266:H874–H880.40. Ota Y, Kugiyama K, Sugiyama S, et al. Impairment of endothelium-dependent relaxation of rabbit aortas by cigarette smoke extract: role of free radicals and attenuation by captopril. Atherosclerosis. 1997. 131:195–202.41. Sugiyama S, Kugiyama K, Ohgushi M, et al. Supersensitivity of atherosclerotic artery to constrictor effect of cigarette smoke extract. Cardiovasc Res. 1998. 38:508–515.42. Kugiyama K, Motoyama T, Hirashima O, et al. Vitamin C attenuates abnormal vasomotor reactivity in spasm coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 1998. 32:103–109.43. Motoyama T, Kawano H, Kugiyama K, et al. Vitamin E administration improves impairment of endothelium-dependent vasodilation in patients with coronary spastic angina. J Am Coll Cardiol. 1998. 32:1672–1679.44. Mackness B, Durrington PN, Mackness MI. Polymorphisms of paraoxonase genes and low-density lipoprotein lipid peroxidation. Lancet. 1999. 353:468–469.45. Itoh T, Yasue H, Yoshimura M, et al. Paraoxonase gene Gln192-Arg (Q192R) polymorphism is associated with coronary artery spasm. Hum Genet. 2002. 110:89–94.46. Shepherd JT, Katsie ZS. Endothelium derived vasoactive factors: I. endothelium-dependent relaxation. Hypertension. 1991. 18:5 Suppl. III76–III85.47. Boulanger C, Luscher TF. Release of endothelin from the porcine aorta: inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990. 85:587–590.48. Takemoto M, Egashira K, Usui M, et al. Important role of tissue angiotensin converting enzyme activity in the pathogenesis of coronary vascular and myocardial structural changes induced by long-term blockade of nitric oxide synthesis in rats. J Clin Invest. 1997. 99:278–287.49. Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2 and prostacyclin. Pharmacol Rev. 1978. 30:293–331.50. Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988. 93:515–524.51. Yanagisawa M, Kurihara H, Kimura S, et al. A novel vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988. 332:411–415.52. Luscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986. 8:344–348.53. Lewis JR, Kisilevsky R, Armstrong PW. Prinzmetal's anfina, normal coronary artery and pericarditis. Can Med Assoc J. 1978. 119:36–39.54. Li JJ, Nie SP, Xu B, Guo YL, Gao Z, Zheng X. Inflammation in variant angina: Is there any evidence? Med Hypotheses. 2007. 68:635–640.55. Kaikita K, Ogawa H, Yasue H, et al. Soluble P-selectin is released into the coronary circulation after coronary spasm. Circulation. 1995. 92:1726–1730.56. Miwa K, Igawa A, Inoue H. Soluble E-selectin, ICAM-1 and VCAM-1 levels in systemic and coronary circulation in patients with variant angina. Cardiovasc Res. 1997. 36:37–44.57. Hung MJ, Cherng WJ, Yang NI, Cheng CW, Li LF. Relation of high-sensitivity C-reactive protein level with coronary vasospastic angina pectoris in patients without hemodynamically significant coronary artery disease. Am J Cardiol. 2005. 96:1484–1490.58. Shimokawa H. Cellular and molecular mechanisms of coronary artery spasm-lessons from animal models. Jpn Circ J. 2000. 64:1–12.59. Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994. 372:231–236.60. Kimura K, Ito M, Amano M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho kinase). Science. 1996. 273:245–248.61. Hirano K, Hirano M, Abe S, et al. Ion channels of vascular smooth muscle cells and endothelial cells. 1991. New-York: Elsevier;93–105.62. Hirano K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharmacol Sci. 2007. 104:109–115.63. Kandabashi T, Shinokawa H, Miyata K, et al. Inhibition of myosin phosphatase by upregulated Rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1β. Circulation. 2000. 101:1319–1323.64. Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002. 105:1545–1547.65. Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998. 273:24266–24271.66. Wolfrum S, Dendorfer A, Rikitake Y, et al. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004. 24:1842–1847.67. Murad F. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006. 355:2003–2011.68. Shimokawa H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular disease. J Cardiovasc Pharmacol. 2002. 39:319–327.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Exercise-Induced Vasospastic Angina With Prominent Regional Wall Motion Abnormality
- The Vasomotor Tone In Vasospastic Angina
- Diagnostic Significance of ECG Ergonovine Provocation Test in Patients with Vasospastic Angina
- Long-term Circadian Patterns of Angina Attacks and Non-pharmacological Provocation Tests Responses in Patients with Vasospastic Angina
- Basal Coronary Artery Tone and Insulin Resistance in Vasospastic Angina