J Korean Med Sci.
2021 Oct;36(40):e259. 10.3346/jkms.2021.36.e259.
Preclinical Evaluation of a Novel Polymer-free Everolimus-eluting Stent in a Mid-term Porcine Coronary Restenosis Model
- Affiliations
-
- 1Department of Cardiology, Chonnam National University Hospital, Gwangju, Korea
- 2Department of Cardiology, Chonnam National University Medical School, Hwasun, Korea
- 3Cardiovascular Research Center, Chonnam National University Hospital, Gwangju, Korea
- 4CGBio Co. Ltd., Seoul, Korea
- KMID: 2521426
- DOI: http://doi.org/10.3346/jkms.2021.36.e259
Abstract
- Background
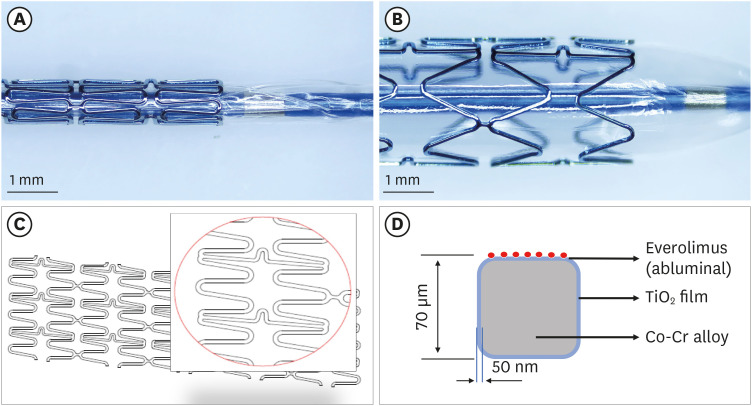
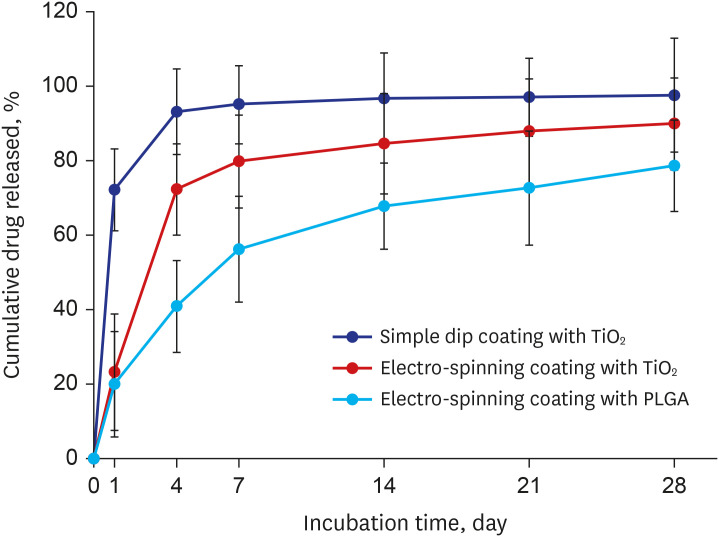
Titanium dioxide films exhibit good biocompatibility and may be effective as drug-binding matrices for drug-eluting stents. We conducted a mid-term evaluation of a novel polymer-free everolimus-eluting stent using nitrogen-doped titanium dioxide film deposition (TIGEREVOLUTION® ) in comparison with a commercial durable polymer everolimus-eluting stent (XIENCE Alpine® ) in a porcine coronary restenosis model.
Methods
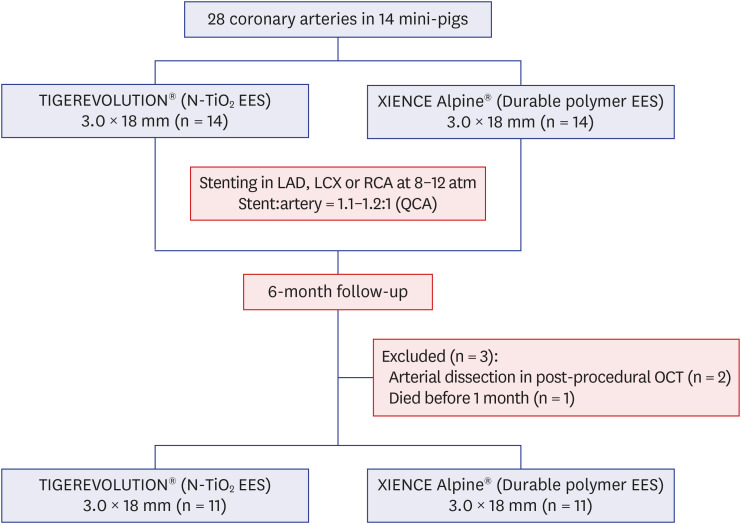
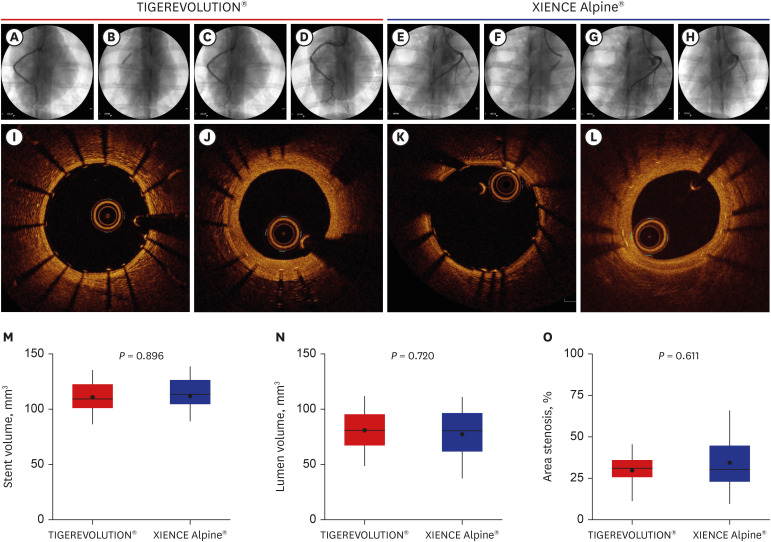
Twenty-eight coronary arteries from 14 mini-pigs were randomly allocated to TIGEREVOLUTION® stent and XIENCE Alpine® stent groups. The stents were implanted in the coronary artery at a 1.1–1.2:1 stent-to-artery ratio. Eleven stented coronary arteries in each group were finally analyzed using coronary angiography, optical coherence tomography, and histopathologic evaluation 6 months after stenting.
Results
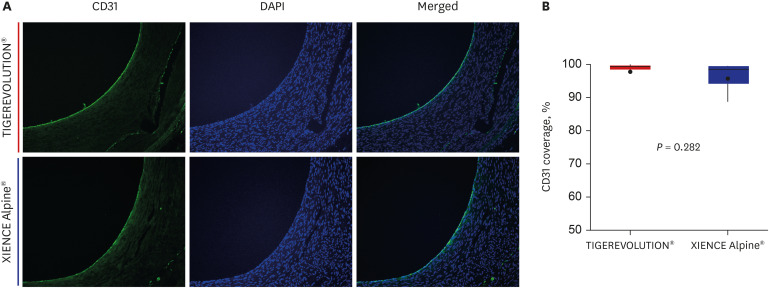
Quantitative coronary analysis showed no significant differences in the preprocedural, post-procedural, and 6-month lumen diameters between the groups. In the volumetric analysis of optical coherence tomography at 6 months, no significant differences were observed in stent volume, lumen volume, and percent area stenosis between the groups. There were no significant differences in injury score, inflammation score, or fibrin score between the groups, although the fibrin score was zero in the TIGEREVOLUTION® stent group (0 vs. 0.07 ± 0.11, P = 0.180).
Conclusion
Preclinical evaluation, including optical coherence tomographic findings 6 months after stenting, demonstrated that the TIGEREVOLUTION® stent exhibited efficacy and safety comparable with the XIENCE Alpine® stent, supporting the need for further clinical studies on the TIGEREVOLUTION® stent.
Figure
Reference
-
1. Gruntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet. 1978; 1(8058):263. PMID: 74678.2. Torrado J, Buckley L, Durán A, Trujillo P, Toldo S, Valle Raleigh J, et al. Restenosis, stent thrombosis, and bleeding complications: navigating between Scylla and Charybdis. J Am Coll Cardiol. 2018; 71(15):1676–1695. PMID: 29650125.3. Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007; 115(11):1440–1455. PMID: 17344324.4. Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007; 27(7):1500–1510. PMID: 17510464.5. Yazdani SK, Vorpahl M, Nakano M, Su SH, Kolodgie FD, Virmani R. In vitro and in vivo characterisation of biodegradable polymer-based drug-eluting stent. EuroIntervention. 2011; 7(7):835–843. PMID: 22082579.
Article6. Nebeker JR, Virmani R, Bennett CL, Hoffman JM, Samore MH, Alvarez J, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006; 47(1):175–181. PMID: 16386683.7. Otsuka Y, Chronos NA, Apkarian RP, Robinson KA. Scanning electron microscopic analysis of defects in polymer coatings of three commercially available stents: comparison of BiodivYsio, Taxus and Cypher stents. J Invasive Cardiol. 2007; 19(2):71–76. PMID: 17268041.8. Song SJ, Park YJ, Park J, Cho MD, Kim JH, Jeong MH, et al. Preparation of a drug-eluting stent using a TiO2 film deposited by plasma enhanced chemical vapour deposition as a drug-combining matrix. J Mater Chem. 2010; 20(23):4792–4801.
Article9. Song SJ, Jung KW, Park YJ, Park J, Cho MD, Jeong MH, et al. Nitrogen-doped TiO2 films as drug-binding matrices for the preparation of drug-eluting stents. J Mater Chem. 2011; 21(22):8169–8177.
Article10. Lim KS, Bae IH, Kim JH, Park DS, Kim JM, Kim JH, et al. Mechanical and histopathological comparison between commercialized and newly designed coronary bare metal stents in a porcine coronary restenosis model. Chonnam Med J. 2013; 49(1):7–13. PMID: 23678471.
Article11. Lim KS, Jeong MH, Bae IH, Park JK, Park DS, Kim JM, et al. Effect of polymer-free TiO2 stent coated with abciximab or alpha lipoic acid in porcine coronary restenosis model. J Cardiol. 2014; 64(5):409–418. PMID: 24852847.
Article12. Bae IH, Lim KS, Park JK, Park DS, Lee SY, Jang EJ, et al. Mechanical behavior and in vivo properties of newly designed bare metal stent for enhanced flexibility. J Ind Eng Chem. 2015; 21:1295–1300.
Article13. Sim DS, Jeong MH, Park DS, Kim JH, Lim KS, Bae IH, et al. A novel polymer-free drug-eluting stent coated with everolimus using nitrogen-doped titanium dioxide film deposition in a porcine coronary restenosis model. Int J Cardiol. 2016; 222:436–440. PMID: 27505330.
Article14. Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992; 19(2):267–274. PMID: 1732351.
Article15. Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998; 31(1):224–230. PMID: 9426044.
Article16. Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, et al. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005; 112(2):270–278. PMID: 15998681.
Article17. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003; 349(14):1315–1323. PMID: 14523139.
Article18. Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004; 350(3):221–231. PMID: 14724301.
Article19. Ong AT, McFadden EP, Regar E, de Jaegere PP, van Domburg RT, Serruys PW. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J Am Coll Cardiol. 2005; 45(12):2088–2092. PMID: 15963413.
Article20. Nordmann AJ, Briel M, Bucher HC. Mortality in randomized controlled trials comparing drug-eluting vs. bare metal stents in coronary artery disease: a meta-analysis. Eur Heart J. 2006; 27(23):2784–2814. PMID: 17020889.
Article21. Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006; 48(12):2584–2591. PMID: 17174201.22. Lagerqvist B, James SK, Stenestrand U, Lindbäck J, Nilsson T, Wallentin L, et al. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007; 356(10):1009–1019. PMID: 17296822.
Article23. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021; 42(14):1289–1367. PMID: 32860058.24. Mauler-Wittwer S, Garot P. The Biolimus A9-coated BioFreedom™ stent: from clinical efficacy to real-world evidence. Future Cardiol. 2021; 17(2):239–255. PMID: 32893680.25. Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015; 373(21):2038–2047. PMID: 26466021.
Article26. Park JK, Lim KS, Bae IH, Nam JP, Cho JH, Choi C, et al. Stent linker effect in a porcine coronary restenosis model. J Mech Behav Biomed Mater. 2016; 53:68–77. PMID: 26318568.
Article27. Hong YJ, Jeong MH, Lee SR, Hong SN, Kim KH, Park HW, et al. Anti-inflammatory effect of abciximab-coated stent in a porcine coronary restenosis model. J Korean Med Sci. 2007; 22(5):802–809. PMID: 17982226.
Article28. Cho JY, Ahn Y, Jeong MH. A bumpy and winding but right path to domestic drug-eluting coronary stents. Korean Circ J. 2013; 43(10):645–654. PMID: 24255648.
Article29. Sim DS, Jeong MH. Development of novel drug-eluting stents for acute myocardial infarction. Chonnam Med J. 2017; 53(3):187–195. PMID: 29026706.
Article30. Nan H, Ping Y, Xuan C, Yongxang L, Xiaolan Z, Guangjun C, et al. Blood compatibility of amorphous titanium oxide films synthesized by ion beam enhanced deposition. Biomaterials. 1998; 19(7-9):771–776. PMID: 9663752.
Article31. Windecker S, Mayer I, De Pasquale G, Maier W, Dirsch O, De Groot P, et al. Stent coating with titanium-nitride-oxide for reduction of neointimal hyperplasia. Circulation. 2001; 104(8):928–933. PMID: 11514381.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Drug-Eluting Stent Strut Fracture as a Cause of Restenosis
- Coronary Stent Thrombosis: Current Insights into New Drug-Eluting Stent Designs
- The Control of Drug Release and Vascular Endothelialization after Hyaluronic Acid-Coated Paclitaxel Multi-Layer Coating Stent Implantation in Porcine Coronary Restenosis Model
- Histopathological Comparison among Biolimus, Zotarolimus and Everolimus-Eluting Stents in Porcine Coronary Restenosis Model
- Systemic drug therapy and restenosis after drug-eluting stent implantation