J Rheum Dis.
2015 Apr;22(2):61-68. 10.4078/jrd.2015.22.2.61.
Pathogenesis of Ankylosing Spondylitis
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea.
- 2Department of Rheumatology, Center of Arthritis and Rheumatism, Kyung Hee University Hospital at Gangdong, Seoul, Korea. boltaguni@yahoo.co.kr
- KMID: 2222907
- DOI: http://doi.org/10.4078/jrd.2015.22.2.61
Abstract
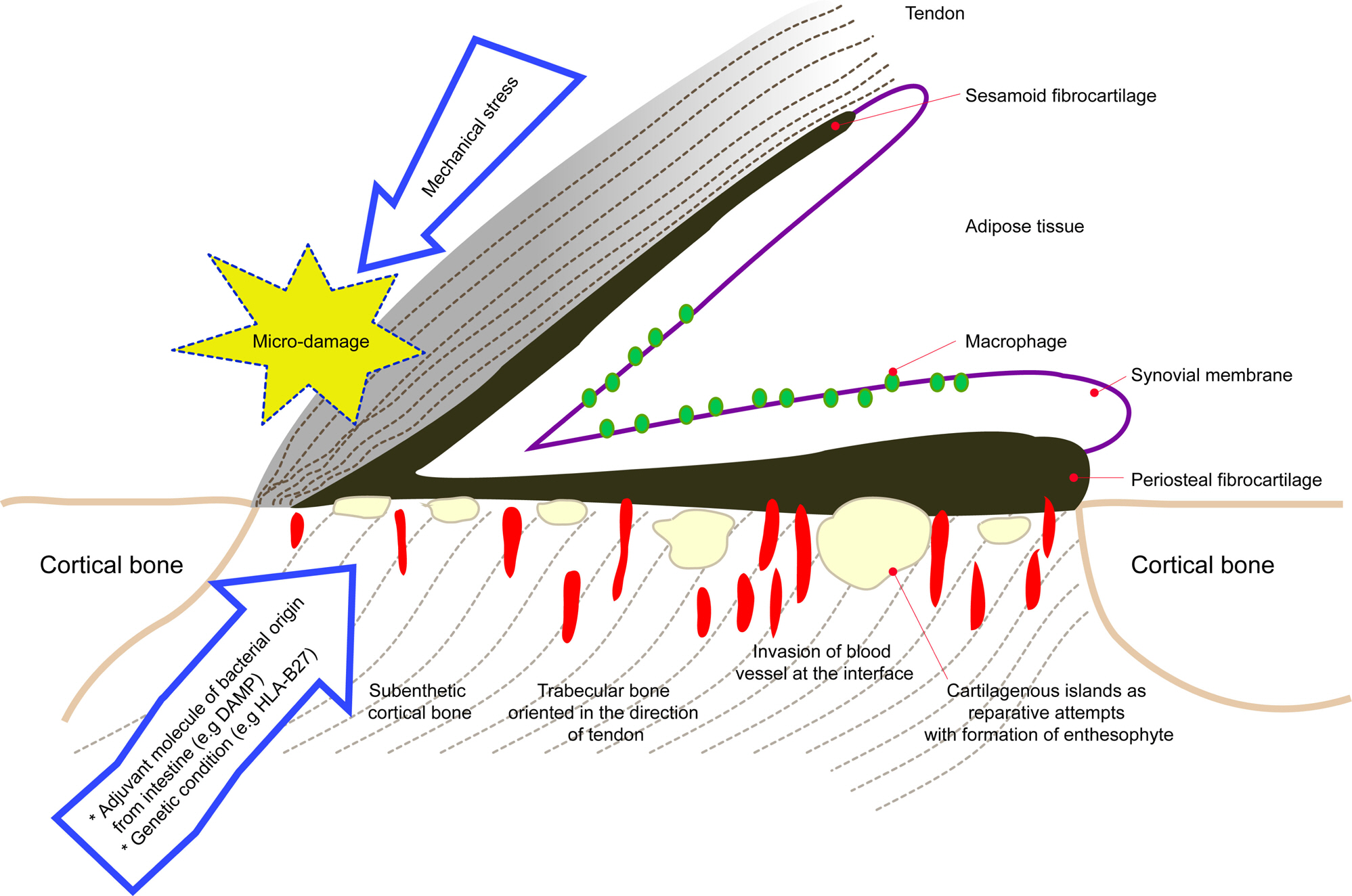
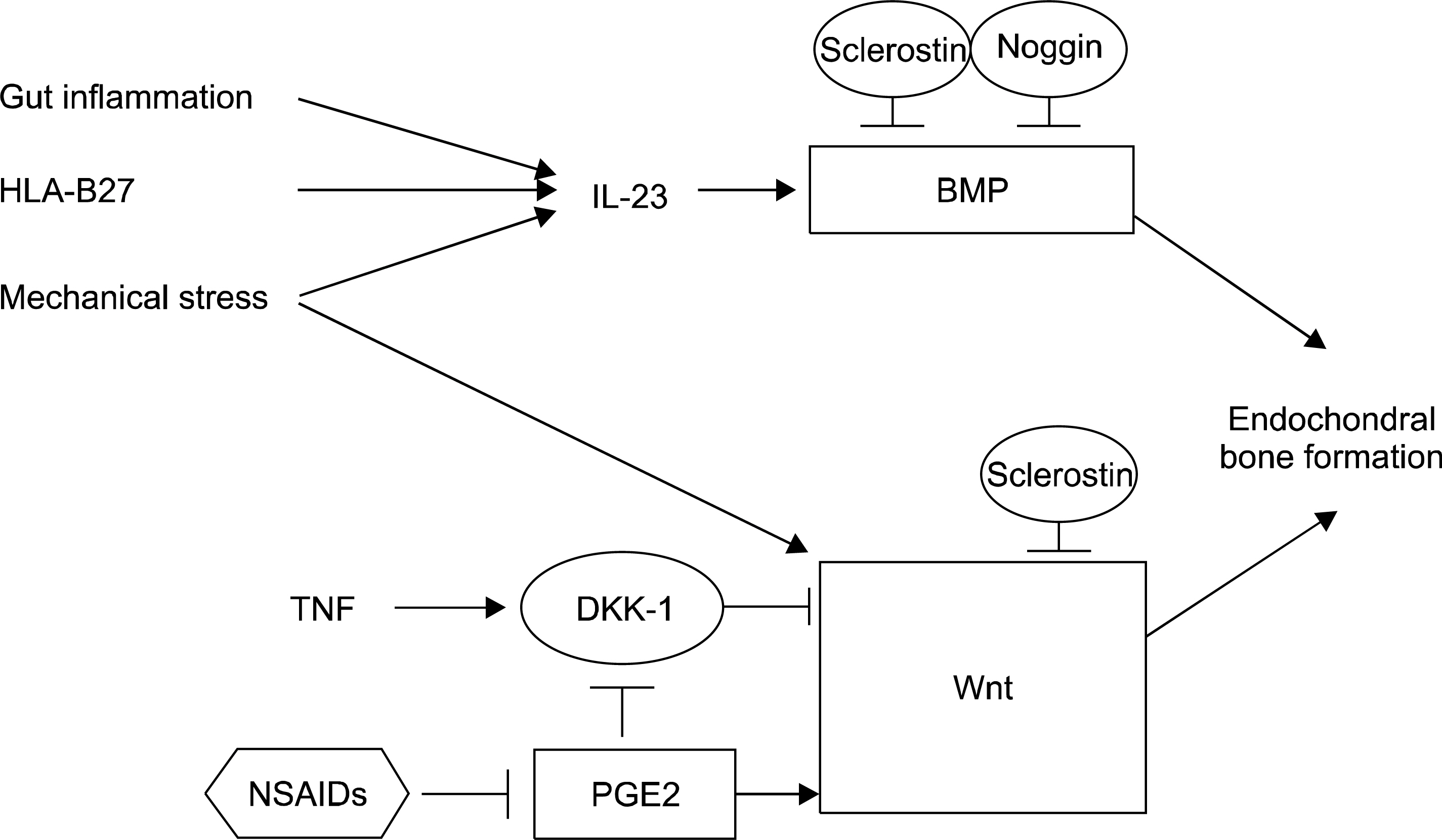
- Ankylosing spondylitis (AS) is the prototype of spondyloarthritis which shares complex clinical phenotypes and risk factors, both genetic and environmental with other chronic inflammatory disease, e.g. inflammatory bowel disease. Human leukocyte antigen-B27 has been known to be the major AS-susceptibility gene for more than 40 years and these molecules have distinct quaternary structures and biogenesis; at least three different hypotheses regarding the contributions to pathogenesis have been proposed. Advances in the discovery of novel susceptibility genes have pointed towards important biological pathways likely responsible for AS pathogenesis. As such, strong involvement of interleukin (IL)-23/IL-17 pathway has been hypothesized. The disease is characterized by inflammation and ankylosis, mainly at the cartilage-bone interface and enthesis. Besides the genetic background, environmental triggers such as microorganisms and mechanical stress are emerging as initiating and perpetuating factors for AS. Current concepts regard new bone formation at the enthesis as a pathological response to biomechanical stress and microbial consequences such as dysbiosis in gut inflammation.
MeSH Terms
Figure
Cited by 1 articles
-
Spontaneous Achilles Tendon Rupture in a Patient with Ankylosing Spondylitis
Seok Kyun Park, Soo Uk Chae
J Rheum Dis. 2016;23(2):136-138. doi: 10.4078/jrd.2016.23.2.136.
Reference
-
1. Tsui FW, Tsui HW, Akram A, Haroon N, Inman RD. The genetic basis of ankylosing spondylitis: new insights into disease pathogenesis. Appl Clin Genet. 2014; 7:105–15.
Article2. Smith JA, Colbert RA. Review: The interleukin-23/ interleukin-17 axis in spondyloarthritis pathogenesis: Th17 and beyond. Arthritis Rheumatol. 2014; 66:231–41.3. Costello ME, Elewaut D, Kenna TJ, Brown MA. Microbes, the gut and ankylosing spondylitis. Arthritis Res Ther. 2013; 15:214.
Article4. Jacques P, McGonagle D. The role of mechanical stress in the pathogenesis of spondyloarthritis and how to combat it. Best Pract Res Clin Rheumatol. 2014; 28:703–10.
Article5. Colbert RA, Rowland-Jones SL, McMichael AJ, Frelinger JA. Allele-specific B pocket transplant in class I major histocompatibility complex protein changes requirement for an-chor residue at P2 of peptide. Proc Natl Acad Sci U S A. 1993; 90:6879–83.
Article6. Allen RL, O'Callaghan CA, McMichael AJ, Bowness P. Cutting edge: HLA-B27 can form a novel beta 2-micro-globulin-free heavy chain homodimer structure. J Immunol. 1999; 162:5045–8.7. Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2014; 57:44–51.
Article8. Ben Dror L, Barnea E, Beer I, Mann M, Admon A. The HLA-B*2705 peptidome. Arthritis Rheum. 2010; 62:420–9.
Article9. Sorrentino R, Böckmann RA, Fiorillo MT. HLA-B27 and antigen presentation: at the crossroads between immune defense and autoimmunity. Mol Immunol. 2014; 57:22–7.
Article10. May E, Dorris ML, Satumtira N, Iqbal I, Rehman MI, Lightfoot E, et al. CD8 alpha beta T cells are not essential to the pathogenesis of arthritis or colitis in HLA-B27 transgenic rats. J Immunol. 2003; 170:1099–105.11. Taurog JD, Dorris ML, Satumtira N, Tran TM, Sharma R, Dressel R, et al. Spondylarthritis in HLA-B27/human be-ta2-microglobulin-transgenic rats is not prevented by lack of CD8. Arthritis Rheum. 2009; 60:1977–84.12. Shaw J, Hatano H, Kollnberger S. The biochemistry and immunology of non-canonical forms of HLA-B27. Mol Immunol. 2014; 57:52–8.
Article13. Thomas GP, Brown MA. Genetics and genomics of ankylosing spondylitis. Immunol Rev. 2010; 233:162–80.
Article14. Colbert RA, DeLay ML, Klenk EI, Layh-Schmitt G. From HLA-B27 to spondyloarthritis: a journey through the ER. Immunol Rev. 2010; 233:181–202.
Article15. Layh-Schmitt G, Yang EY, Kwon G, Colbert RA. HLA-B27 alters the response to tumor necrosis factor α and promotes osteoclastogenesis in bone marrow monocytes from HLA-B27-transgenic rats. Arthritis Rheum. 2013; 65:2123–31.
Article16. Ciccia F, Accardo-Palumbo A, Rizzo A, Guggino G, Raimondo S, Giardina A, et al. Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann Rheum Dis. 2014; 73:1566–74.
Article17. De Cata A, Inglese M, Rubino R, Molinaro F, Mazzoccoli G. The synovio-entheseal complex in enthesoarthritis. Clin Exp Med. 2015; Feb 12 [Epub].
Article18. Lories RJ, Derese I, Luyten FP. Modulation of bone morpho-genetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005; 115:1571–9.
Article19. Sieper J, Appel H, Braun J, Rudwaleit M. Critical appraisal of assessment of structural damage in ankylosing spondylitis: implications for treatment outcomes. Arthritis Rheum. 2008; 58:649–56.
Article20. van Duivenvoorde LM, Dorris ML, Satumtira N, van Tok MN, Redlich K, Tak PP, et al. Relationship between inflammation, bone destruction, and osteoproliferation in the HLA-B27/human β 2-microglobulin-transgenic rat model of spondylarthritis. Arthritis Rheum. 2012; 64:3210–9.21. Baraliakos X, Haibel H, Listing J, Sieper J, Braun J. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis. 2014; 73:710–5.22. Hreggvidsdottir HS, Noordenbos T, Baeten DL. Inflammatory pathways in spondyloarthritis. Mol Immunol. 2014; 57:28–37.
Article23. Sonomoto K, Yamaoka K, Oshita K, Fukuyo S, Zhang X, Nakano K, et al. Interleukin-1β induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2 pathway. Arthritis Rheum. 2012; 64:3355–63.
Article24. van Duivenvoorde LM, Ambarus CA, Masdar H, van Tok MN, Tak PP, Yeremenko NG, et al. A2. 15 relative overexpression of transmembrane versus soluble TNF in human and experimental spondyloarthritis. Ann Rheum Dis. 2013; 72:A9–10.25. Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-γ t+ CD3+CD4-CD8-entheseal resident T cells. Nat Med. 2012; 18:1069–76.26. Kroon F, Landewé R, Dougados M, van der Heijde D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012; 71:1623–9.
Article27. Lories RJ, Luyten FP, de Vlam K. Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res Ther. 2009; 11:221.
Article28. Baraliakos X, Listing J, Rudwaleit M, Sieper J, Braun J. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res Ther. 2008; 10:R104.
Article29. McGonagle D, Stockwin L, Isaacs J, Emery P. An enthesitis based model for the pathogenesis of spondyloarthropathy. additive effects of microbial adjuvant and biomechanical factors at disease sites. J Rheumatol. 2001; 28:2155–9.30. McGonagle D, Lories RJ, Tan AL, Benjamin M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 2007; 56:2482–91.
Article31. Shah N, Morsi Y, Manasseh R. From mechanical stimulation to biological pathways in the regulation of stem cell fate. Cell Biochem Funct. 2014; 32:309–25.
Article32. Maksymowych WP, Morency N, Conner-Spady B, Lambert RG. Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: evidence for a window of opportunity in disease modification. Ann Rheum Dis. 2013; 72:23–8.
Article33. Santos A, Bakker AD, Zandieh-Doulabi B, Semeins CM, Klein-Nulend J. Pulsating fluid flow modulates gene expression of proteins involved in Wnt signaling pathways in osteocytes. J Orthop Res. 2009; 27:1280–7.
Article34. Haudenschild AK, Hsieh AH, Kapila S, Lotz JC. Pressure and distortion regulate human mesenchymal stem cell gene expression. Ann Biomed Eng. 2009; 37:492–502.
Article35. Lories RJ, Derese I, de Bari C, Luyten FP. Evidence for uncoupling of inflammation and joint remodeling in a mouse model of spondylarthritis. Arthritis Rheum. 2007; 56:489–97.
Article36. Jacques P, Lambrecht S, Verheugen E, Pauwels E, Kollias G, Armaka M, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis. 2014; 73:437–45.
Article37. Daoussis D, Liossis SN, Solomou EE, Tsanaktsi A, Bounia K, Karampetsou M, et al. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 2010; 62:150–8.
Article38. Klingberg E, Nurkkala M, Carlsten H, Forsblad-d'Elia H. Biomarkers of bone metabolism in ankylosing spondylitis in relation to osteoproliferation and osteoporosis. J Rheumatol. 2014; 41:1349–56.
Article39. Chan AT, Kollnberger SD, Wedderburn LR, Bowness P. Expansion and enhanced survival of natural killer cells expressing the killer immunoglobulin-like receptor KIR3DL2 in spondylarthritis. Arthritis Rheum. 2005; 52:3586–95.
Article40. DeLay ML, Turner MJ, Klenk EI, Smith JA, Sowders DP, Colbert RA. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009; 60:2633–43.
Article41. Appel H, Maier R, Bleil J, Hempfing A, Loddenkemper C, Schlichting U, et al. In situ analysis of interleukin-23- and interleukin-12-positive cells in the spine of patients with ankylosing spondylitis. Arthritis Rheum. 2013; 65:1522–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ankylosing Spondylitis: Prevention And Surgical Correction Of Deformity
- Treatment of Cervical Cord Injury with Ankylosing Spondylitis: Case Report
- Orthopaedic Management of Ankylosing Spondylitis
- Clinieal Values of Single Photon Emission Computed Tomography ( SPECT ) in Ankylosing Spondylitis
- Treatment of Cervical Cord Injury in Ankylosing Spondylitis