Korean Diabetes J.
2010 Jun;34(3):200-206. 10.4093/kdj.2010.34.3.200.
Homocysteine as a Risk Factor for Development of Microalbuminuria in Type 2 Diabetes
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. kulee@amc.seoul.kr
- KMID: 2222376
- DOI: http://doi.org/10.4093/kdj.2010.34.3.200
Abstract
- BACKGROUND
Kidney function is critical in homocysteine clearance, and plasma homocysteine level is frequently increased in patients with renal failure. On the other hand, recent studies in animals have shown that hyperhomocysteinemia induces renal injury. In this study, we determined whether hyperhomocysteinemia can be a risk factor for the development of microalbuminuria in patients with type 2 diabetes.
METHODS
A nested case-control study. Of 887 patients with type 2 diabetes who did not have microalbuminuria at baseline, 76 developed microalbuminuria during follow-up (mean, 36.0 +/- 11.7 months; range, 18 to 76 months). The control group consisted of 152 age- and sex-matched subjects who did not develop microalbuminuria. Baseline plasma homocysteine concentrations were measured in stored samples.
RESULTS
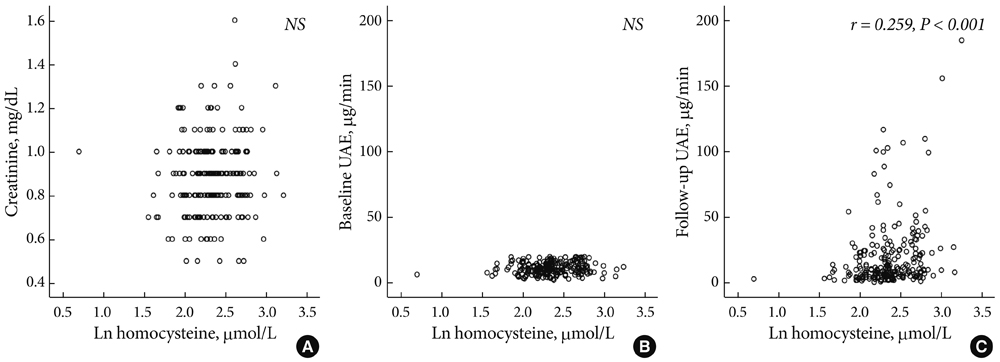
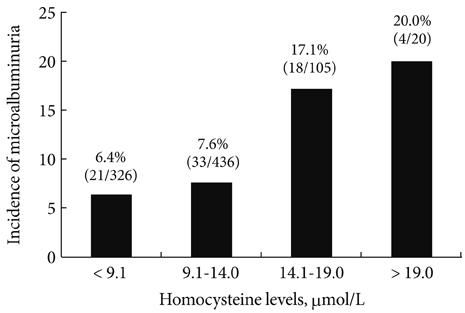
Baseline plasma homocysteine concentrations and mean HbA1C levels during follow-up were significantly higher in patients who developed microalbuminuria than in those who remained normoalbuminuric. Multivariate logistic regression analysis showed that baseline plasma homocysteine level and mean HbA1C were independent predictors of microalbuminuria in type 2 diabetes.
CONCLUSION
Hyperhomocysteinemia was associated with increased risk of microalbuminuria in patients with type 2 diabetes supporting the concept that hyperhomocysteinemia has an etiologic role in the pathogenesis of diabetic nephropathy.
Keyword
MeSH Terms
Figure
Reference
-
1. Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984. 310:356–360.2. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001. 286:421–426.3. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000. 321:405–412.4. Gall MA, Hougaard P, Borch-Johnsen K, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ. 1997. 314:783–788.5. Park JY, Kim HK, Chung YE, Kim SW, Hong SK, Lee KU. Incidence and determinants of microalbuminuria in Koreans with type 2 diabetes. Diabetes Care. 1998. 21:530–534.6. Hoffer LJ. Testing the homocysteine hypothesis in end-stage renal disease: problems and a possible solution. Kidney Int. 2006. 69:1507–1510.7. Ruan L, Chen W, Srinivasan SR, Xu J, Toprak A, Berenson GS. Plasma homocysteine is adversely associated with glomerular filtration rate in asymptomatic black and white young adults: the Bogalusa heart study. Eur J Epidemiol. 2009. 24:315–319.8. Kumagai H, Katoh S, Hirosawa K, Kimura M, Hishida A, Ikegaya N. Renal tubulointerstitial injury in weanling rats with hyperhomocysteinemia. Kidney Int. 2002. 62:1219–1228.9. Yi F, dos Santos EA, Xia M, Chen QZ, Li PL, Li N. Podocyte injury and glomerulosclerosis in hyperhomocysteinemic rats. Am J Nephrol. 2007. 27:262–268.10. Ingram AJ, Krepinsky JC, James L, Austin RC, Tang D, Salapatek AM, Thai K, Scholey JW. Activation of mesangial cell mapk in response to homocysteine. Kidney Int. 2004. 66:733–745.11. Yi F, Zhang AY, Li N, Muh RW, Fillet M, Renert AF, Li PL. Inhibition of ceramide-redox signaling pathway blocks glomerular injury in hyperhomocysteinemic rats. Kidney Int. 2006. 70:88–96.12. Hwang SY, Woo CW, Au-Yeung KK, Siow YL, Zhu TY, O K. Homocysteine stimulates monocyte chemoattractant protein-1 expression in the kidney via nuclear factor-kappaB activation. Am J Physiol Renal Physiol. 2008. 294:F236–F244.13. Looker HC, Fagot-Campagna A, Gunter EW, Pfeiffer CM, Narayan KM, Knowler WC, Hanson RL. Homocysteine as a risk factor for nephropathy and retinopathy in type 2 diabetes. Diabetologia. 2003. 46:766–772.14. Jager A, Kostense PJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Donker AJ, Stehouwer CD. Serum homocysteine levels are associated with the development of (micro)albuminuria: The Hoorn Study. Arterioscler Thromb Vasc Biol. 2001. 21:74–81.15. Chico A, Perez A, Cordoba A, Arcelus R, Carreras G, de Leiva A, Gonzalez-Sastre F, Blanco-Vaca F. Plasma homocysteine is related to albumin excretion rate in patients with diabetes mellitus: a new link between diabetic nephropathy and cardiovascular disease? Diabetologia. 1998. 41:684–693.16. Wollesen F, Brattstrom L, Refsum H, Ueland PM, Berglund L, Berne C. Plasma total homocysteine and cysteine in relation to glomerular filtration rate in diabetes mellitus. Kidney Int. 1999. 55:1028–1035.17. Davies L, Wilmshurst EG, McElduff A, Gunton J, Clifton-Bligh P, Fulcher GR. The relationship among homocysteine, creatinine clearance, and albuminuria in patients with type 2 diabetes. Diabetes Care. 2001. 24:1805–1809.18. Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007. 18:1353–1361.19. Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003. 63:225–232.20. Starkebaum G, Harlan JM. Endothelial cell injury due to coppercatalyzed hydrogen peroxide generation from homocysteine. J Clin Invest. 1986. 77:1370–1376.21. Woo KS, Chook P, Lolin YI, Cheung AS, Chan LT, Sun YY, Sanderson JE, Metreweli C, Celermajer DS. Hyperhomocyst(e) inemia is a risk factor for arterial endothelial dysfunction in humans. Circulation. 1997. 96:2542–2544.22. Castro R, Rivera I, Blom HJ, Jakobs C, Tavares de Almeida I. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: an overview. J Inherit Metab Dis. 2006. 29:3–20.23. Hoogeveen EK, Kostense PJ, Jager A, Heine RJ, Jakobs C, Bouter LM, Donker AJ, Stehouwer CD. Serum homocysteine level and protein intake are related to risk of microalbuminuria: the Hoorn Study. Kidney Int. 1998. 54:203–209.24. Locatelli F, Pozzoni P, Del Vecchio L. Renal manifestations in the metabolic syndrome. J Am Soc Nephrol. 2006. 17:S81–S85.25. Lee KU. Oxidative stress markers in Korean subjects with insulin resistance syndrome. Diabetes Res Clin Pract. 2001. 54:Suppl 2. S29–S33.26. Bjorck J, Hellgren M, Rastam L, Lindblad U. Associations between serum insulin and homocysteine in a Swedish population-a potential link between the metabolic syndrome and hyperhomocysteinemia: the Skaraborg project. Metabolism. 2006. 55:1007–1013.27. Ernster VL. Nested case-control studies. Prev Med. 1994. 23:587–590.28. Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004. 351:2599–2610.29. Clarke R, Refsum H, Birks J, Evans JG, Johnston C, Sherliker P, Ueland PM, Schneede J, McPartlin J, Nexo E, Scott JM. Screening for vitamin B-12 and folate deficiency in older persons. Am J Clin Nutr. 2003. 77:1241–1247.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diabetic Nephropathy in Childhood and Adolescence (I) : Clinical Features
- Lack of Effectiveness of Glomerular Hyperfiltration on Development of Microalbuminuria in Type 2 Diabetic Patients: five Year Follow-up Study
- Frequencies and Risk Factors for Microvascular Complications in Patients with Type 1 Diabetes Mellitus
- ACE Gene Polymorphism and the Development of Microalbuminura in Korean Type 2 Diabetes Patients
- Relationship between plasma homocysteine levels and chronic diabetic complications in NIDDM patients