Allergy Asthma Respir Dis.
2016 Jan;4(1):14-21. 10.4168/aard.2016.4.1.14.
Relationships between fractional exhaled nitric oxide levels and FEF25%-75% in children with asthma
- Affiliations
-
- 1Department of Pediatrics, Korea University College of Medicine, Seoul, Korea. yoolina@korea.ac.kr
- 2Environmental Health Center, Korea University Anam Hospital, Seoul, Korea.
- 3Allergy Immunology Center, Korea University, Seoul, Korea.
- KMID: 2218614
- DOI: http://doi.org/10.4168/aard.2016.4.1.14
Abstract
- PURPOSE
Fractional exhaled nitric oxide (FeNO) is considered an indirect marker of airway inflammation, and forced expiratory flow between 25% and 75% of vital capacity (FEF25%-75%) is widely used as a sensitive indicator of small airway obstruction in asthma. The aim of this study was to investigate relationships between FeNO and FEF25%-75% in children with asthma.
METHODS
A total of 118 children with asthma underwent spirometry and measurement of eosinophil markers. FeNO levels were measured, and skin prick tests to 13 common allergens were done. Study subjects were divided into 2 groups according to FEF25%-75% values (group 1, normal FEF25%-75%> or =65%pred, n=90; group 2, impaired FEF25%-75%<65%pred, n=28).
RESULTS
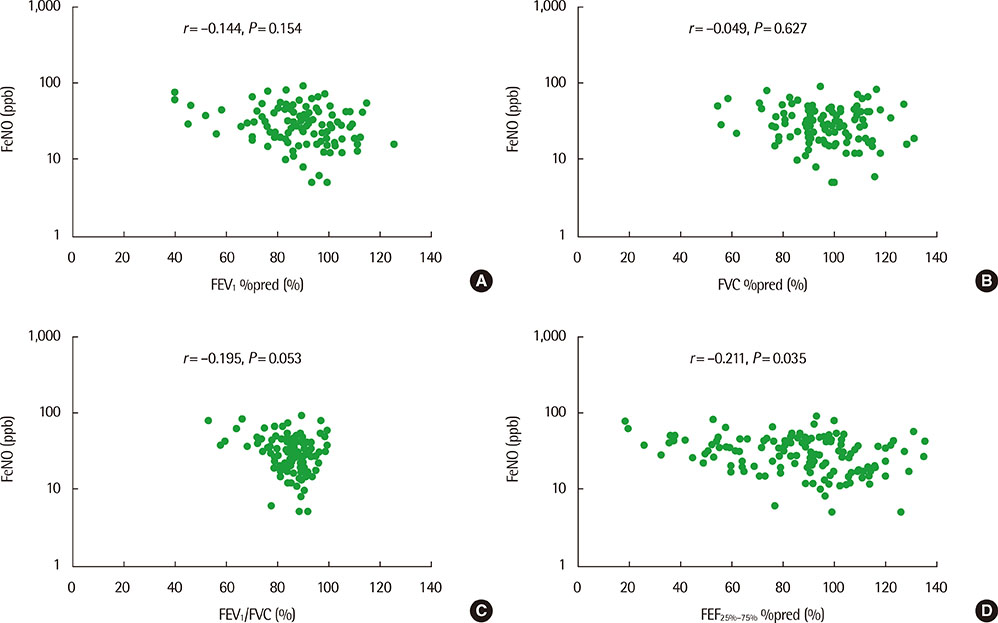
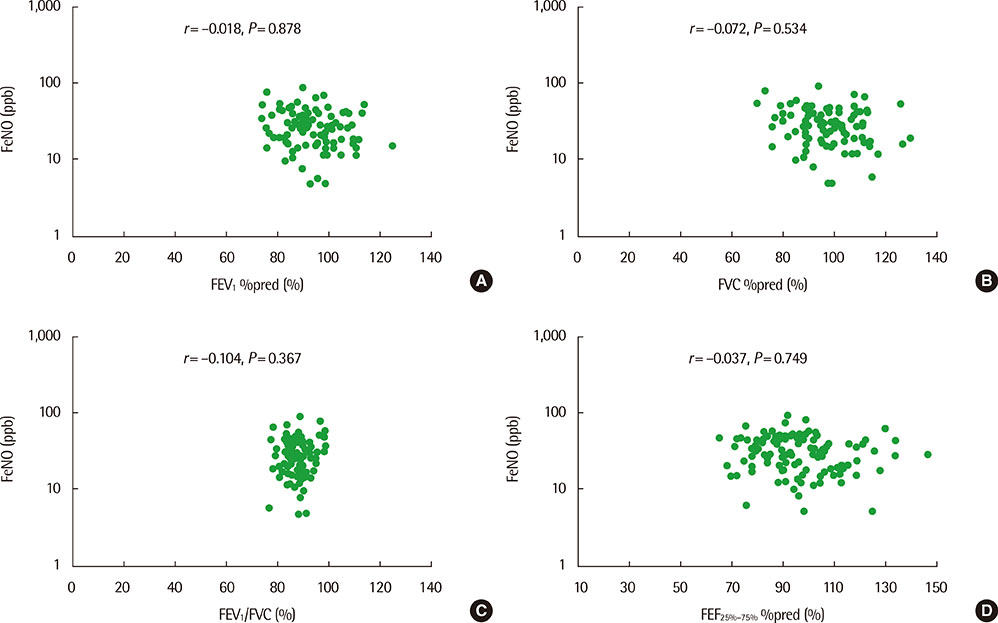
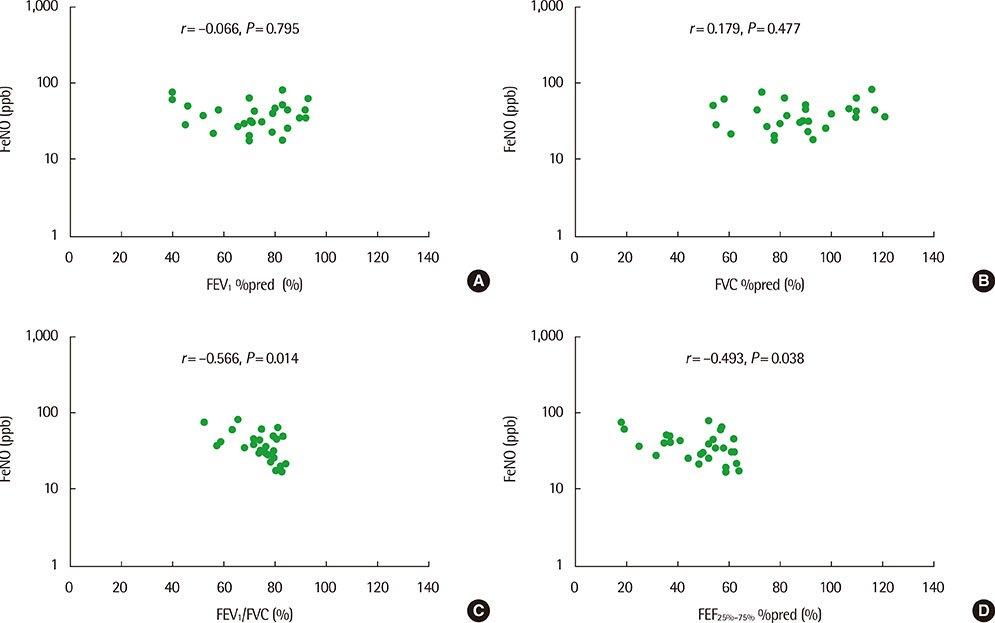
The mean (+/-standard deviation, SD) age was not significantly different between groups 1 and 2 (10.3+/-2.8 years vs. 11.1+/-3.4 years), and the sex ratio was also not significantly different between 2 groups. The geometric mean (range of 1 SD) concentration of FeNO was significantly higher in group 2 than in group 1 (25.8 ppb [14.2-46.9 ppb] vs. 37.2 ppb [24.2-57.2 ppb], P=0.008). A significant inverse correlation between FeNO and FEF25%-75% was observed in group 2 (r=-0.493, P=0.038), but not in group 1 (r=-0.037, P=0.749) after adjustment for confounders, such as atopy, age, sex, weight, and height.
CONCLUSION
FeNO levels were higher in group of asthmatic children with impaired FEF25%-75% level. FeNO levels were inversely correlated with FEF 25%-75% only in impaired small-airway obstruction group after adjustment for atopy. These results suggest that small-airway obstruction may relate more closely to airway inflammation in asthmatic children with impaired small-airway function.
Keyword
MeSH Terms
Figure
Reference
-
1. International consensus report on diagnosis and treatment of asthma. National Heart, Lung, and Blood Institute, National Institutes of Health. Bethesda, Maryland 20892. Publication no. 92-3091, March 1992. Eur Respir J. 1992; 5:601–641.2. Pyun BY. Guideline for the management of childhood asthma. J Korean Pediatr Soc. 2001; 44:727–731.3. Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF Jr, Sorkness CA. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004; 170:426–432.4. McFadden ER Jr, Linden DA. A reduction in maximum mid-expiratory flow rate. a spirographic manifestation of small airway disease. Am J Med. 1972; 52:725–737.5. Gibb ER, Thyne SM, Kaplan DN, Ly NP. Asthma, FEF25–75, and hospitalizations in children. Pediatr Allergy Immunol Pulmonol. 2013; 26:115–121.6. Barnes PJ, Liew FY. Nitric oxide and asthmatic inflammation. Immunol Today. 1995; 16:128–130.
Article7. Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993; 6:1368–1370.8. Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998; 53:91–95.
Article9. Mattes J, Storm van's Gravesande K, Reining U, Alving K, Ihorst G, Henschen M, et al. NO in exhaled air is correlated with markers of eosinophilic airway inflammation in corticosteroid-dependent childhood asthma. Eur Respir J. 1999; 13:1391–1395.
Article10. Ko HS, Chung SH, Choi YS, Choi SH, Rha YH. Relationship between exhaled nitric oxide and pulmonary function test in children with asthma. Korean J Pediatr. 2008; 51:181–187.
Article11. Choi BS, Jee HM, Park YH, Kim KW, Sohn MH, Kim KE. Relationship between exhaled nitric oxide concentration and pulmonary function/airway hyperresponsiveness in asthmatic children. Pediatr Allergy Respir Dis. 2009; 19:291–299.12. Colon-Semidey AJ, Marshik P, Crowley M, Katz R, Kelly HW. Correlation between reversibility of airway obstruction and exhaled nitric oxide levels in children with stable bronchial asthma. Pediatr Pulmonol. 2000; 30:385–392.
Article13. Steerenberg PA, Janssen NA, de Meer G, Fischer PH, Nierkens S, van Loveren H, et al. Relationship between exhaled NO, respiratory symptoms, lung function, bronchial hyperresponsiveness, and blood eosinophilia in school children. Thorax. 2003; 58:242–245.
Article14. Kim JO, Woo SI, Hahn YS. Relevance of exhaled nitric oxide levels to asthma control test scores and spirometry values in children with atopic asthma. Pediatr Allergy Respir Dis. 2011; 21:24–31.
Article15. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007; 120:5 Suppl. S94–S138.16. Cockcroft DW. Bronchoprovocation methods: direct challenges. Clin Rev Allergy Immunol. 2003; 24:19–26.
Article17. Ciprandi G, Tosca MA, Cirillo I, Lionetti E, Leonardi S, Miraglia Del Giudice M, et al. Impaired FEF25-75 may predict high exhaled nitric oxide values in children with allergic rhinitis and/or asthma. J Biol Regul Homeost Agents. 2012; 26:1 Suppl. S27–S33.18. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995; 152:1107–1136.19. Park CH, Kim HB, Jung YH, Lee E, Yang SI, Seo JH, et al. Predicted normal values of pulmonary function tests in normal Korean children. Allergy Asthma Respir Dis. 2014; 2:187–193.
Article20. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.
Article21. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615.
Article22. Johansson SG, Yman L. In vitro assays for immunoglobulin E. Methodology, indications, and interpretation. Clin Rev Allergy. 1988; 6:93–139.23. del Giudice MM, Brunese FP, Piacentini GL, Pedulla M, Capristo C, Decimo F, et al. Fractional exhaled nitric oxide (FENO), lung function and airway hyperresponsiveness in naïve atopic asthmatic children. J Asthma. 2004; 41:759–765.
Article24. Covar RA, Szefler SJ, Martin RJ, Sundstrom DA, Silkoff PE, Murphy J, et al. Relations between exhaled nitric oxide and measures of disease activity among children with mild-to-moderate asthma. J Pediatr. 2003; 142:469–475.
Article25. Sampson AP. The role of eosinophils and neutrophils in inflammation. Clin Exp Allergy. 2000; 30:Suppl 1. 22–27.
Article26. Prasad A, Langford B, Stradling JR, Ho LP. Exhaled nitric oxide as a screening tool for asthma in school children. Respir Med. 2006; 100:167–173.
Article27. Joseph-Bowen J, de Klerk N, Holt PG, Sly PD. Relationship of asthma, atopy, and bronchial responsiveness to serum eosinophil cationic proteins in early childhood. J Allergy Clin Immunol. 2004; 114:1040–1045.
Article28. Paredi P, Kharitonov SA, Meah S, Barnes PJ, Usmani OS. A novel approach to partition central and peripheral airway nitric oxide. Chest. 2014; 145:113–119.
Article29. Pedroletti C, Zetterquist W, Nordvall L, Alving K. Evaluation of exhaled nitric oxide in schoolchildren at different exhalation flow rates. Pediatr Res. 2002; 52:393–398.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fractional exhaled nitric oxide and forced expiratory flow between 25% and 75% of vital capacity in children with controlled asthma
- Measurements of fractional exhaled nitric oxide in pediatric asthma
- Utility of Fractional Exhaled Nitric Oxide in the Diagnosis of Asthma and the Assessment of Asthma Control
- Relevance of Exhaled Nitric Oxide Levels to Asthma Control Test Scores and Spirometry Values in Children with Atopic Asthma
- Measurement and Interpretation of Fractional Exhaled Nitric Oxide