J Korean Ophthalmol Soc.
2013 Apr;54(4):610-617. 10.3341/jkos.2013.54.4.610.
Predictive Factors for Visual Outcome after Treatment for Myopic Choroidal Neovascularization
- Affiliations
-
- 1Department of Ophthalmology, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea. ihyun@inje.ac.kr
- 2Department of Ophthalmology, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- KMID: 2216942
- DOI: http://doi.org/10.3341/jkos.2013.54.4.610
Abstract
- PURPOSE
To determine predictive factors associated with visual outcome after treatment for myopic choroidal neovascularization (mCNV).
METHODS
Medical records of the patients who underwent photodynamic therapy (PDT), intravitreal anti-vascular endothelial growth factor (Anti-VEGF) injection, or combination therapy of PDT and Anti-VEGF for myopic CNV, and followed up for more than a year, were reviewed retrospectively. Multiple linear regression analyses were performed to evaluate the predictive factors significantly associated with the visual outcome at 1 year after the treatment.
RESULTS
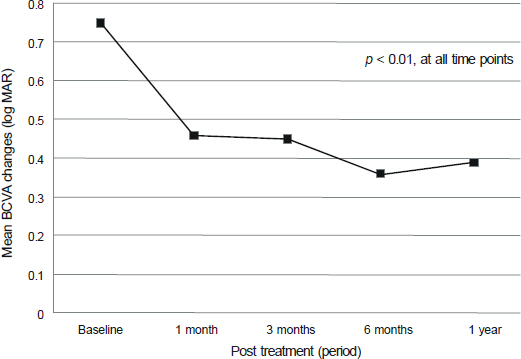
Mean best-corrected visual acuity (BCVA) of 45 eyes of 45 patients showed statistically significant improvement 1 year after the treatment with a mean of 3.5 line improvement (p < 0.01, Wilcoxon signed rank test). Age, 1-month BCVA after treatment and treatment type appeared to be associated with the 1-year visual outcome after treatment for mCNV (p = 0.033, p < 0.001, and p = 0.044, respectively, multivariate linear regression analysis).
CONCLUSIONS
Younger age (less than 40 years), better 1-month BCVA after treatment, intravitreal Anti-VEGF monotherapy were associated with improved visual outcome after treatment for mCNV. In particular, 1-month BCVA after treatment is a useful indicator to predict therapeutic response after treatment for mCNV.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina. 1992; 12:127–33.
Article2. Hotchkiss ML, Fine SL. Pathologic myopia and choroidal neovascularization. Am J Ophthalmol. 1981; 91:177–83.
Article3. Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol. 2003; 87:570–3.
Article4. Bottoni F, Tilanus M. The natural history of juxtafoveal and subfoveal choroidal neovascularization in high myopia. Int Ophthalmol. 2001; 24:249–55.5. Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology. 2003; 110:1297–305.6. Yoshida T, Ohno-Matsui K, Ohtake Y, et al. Long-term visual prognosis of choroidal neovascularization in high myopia: a comparison between age groups. Ophthalmology. 2002; 109:712–9.7. Blinder KJ, Blumenkranz MS, Bressler NM, et al. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial - VIP report no. 3. Ophthalmology. 2003; 110:667–73.8. Lam DS, Chan WM, Liu DT, et al. Photodynamic therapy with verteporfin for subfoveal choroidal neovascularisation of pathologic myopia in Chinese eyes: a prospective series of 1 and 2 year follow up. Br J Ophthalmol. 2004; 88:1315–9.
Article9. Schnurrbusch UE, Jochmann C, Wiedemann P, Wolf S. Quantitative assessment of the long-term effect of photodynamic therapy in patients with pathologic myopia. Graefes Arch Clin Exp Ophthalmol. 2005; 243:829–33.
Article10. Pece A, Isola V, Vadalà M, Matranga D. Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization secondary to pathologic myopia: long-term study. Retina. 2006; 26:746–51.11. Chen YS, Lin JY, Tseng SY, et al. Photodynamic therapy for Taiwanese patients with pathologic myopia: a 2-year follow-up. Retina. 2007; 27:839–45.12. Cohen SY. Anti-VEGF drugs as the 2009 first-line therapy for choroidal neovascularization in pathologic myopia. Retina. 2009; 29:1062–6.
Article13. Byeon SH, Kwon OW, Lee SC, et al. Indocyanine green angio-graphic features of myopic subfoveal choroidal neovascularization as a prognostic factor after photodynamic therapy. Korean J Ophthalmol. 2006; 20:18–25.
Article14. Kuo JZ, Ong FS, Yeung L, et al. Predictive factors for visual outcome to intravitreal bevacizumab in young Chinese patients with myopic choroidal neovascularization. Retina. 2011; 31:1835–40.
Article15. Yoon JU, Kim YM, Lee SJ, et al. Prognostic factors for visual outcome after intravitreal anti-vegf injection for naive myopic choroidal neovascularization. Retina. 2012; [Epub ahead of print].
Article16. Axer-Siegel R, Ehrlich R, Yassur Y, et al. Photodynamic therapy for age-related macular degeneration in a clinical setting: visual results and angiographic patterns. Am J Ophthalmol. 2004; 137:258–64.
Article17. Hampton GR, Kohen D, Bird AC. Visual prognosis of disciform degeneration in myopia. Ophthalmology. 1983; 90:923–6.
Article18. Secrétan M, Kuhn D, Soubrane G, Coscas G. Long-term visual outcome of choroidal neovascularization in pathologic myopia: natural history and laser treatment. Eur J Ophthalmol. 1997; 7:307–16.
Article19. Brancato R, Pece A, Avanza P, Radrizzani E. Photocoagulation scar expansion after laser therapy for choroidal neovascularization in degenerative myopia. Retina. 1990; 10:239–43.
Article20. Pece A, Brancato R, Avanza P, et al. Laser photocoagulation of choroidal neovascularization in pathologic myopia: long-term results. Int Ophthalmol. 1994-1995; 18:339–44.
Article21. Ruiz-Moreno JM, Montero JA. Long-term visual acuity after argon green laser photocoagulation of juxtafoveal choroidal neovascularization in highly myopic eyes. Eur J Ophthalmol. 2002; 12:117–22.
Article22. Virgili G, Varano M, Giacomelli G, et al. Photodynamic therapy for nonsubfoveal choroidal neovascularization in 100 eyes with pathologic myopia. Am J Ophthalmol. 2007; 143:77–82.
Article23. Pece A, Vadalà M, Isola V, Matranga D. Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization in pathologic myopia: a long-term follow-up study. Am J Ophthalmol. 2007; 143:449–54.
Article24. Gibson J. Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularisation secondary to pathological myopia. Eye (Lond). 2005; 19:829–30.
Article25. Lam DS, Liu DT, Fan DS, et al. Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization secondary to pathologic myopia-1-year results of a prospective series. Eye (Lond). 2005; 19:834–40.
Article26. Gelisken F, Inhoffen W, Hermann A, et al. Verteporfin photodynamic therapy for extrafoveal choroidal neovascularisation in pathologic myopia. Graefes Arch Clin Exp Ophthalmol. 2004; 242:926–30.
Article27. Chan WM, Lai TY, Wong AL, et al. Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of choroidal neovascularisation secondary to pathological myopia: a pilot study. Br J Ophthalmol. 2007; 91:174–9.
Article28. Marticorena J, Gomez-Ulla F, Fernandez M, et al. Combined photodynamic therapy and intravitreal triamcinolone acetonide for the treatment of myopic subfoveal choroidal neovascularization. Am J Ophthalmol. 2006; 142:335–7.
Article29. Rishi P, Rishi E, Venkataraman A, et al. Photodynamic mono-therapy or combination treatment with intravitreal triamcinolone acetonide, bevacizumab or ranibizumab for choroidal neovascularization associated with pathological myopia. Indian J Ophthalmol. 2011; 59:242–6.
Article30. Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin). Retina. 2006; 26:257–61.
Article31. Gharbiya M, Giustolisi R, Allievi F, et al. Choroidal neovascularization in pathologic myopia: intravitreal ranibizumab versus bevacizumab-a randomized controlled trial. Am J Ophthalmol. 2010; 149:458–64.
Article32. Matsuo M, Honda S, Matsumiya W, et al. Comparison between anti-vascular endothelial growth factor therapy and photodynamic therapy for myopic choroidal neovascularization. Eur J Ophthalmol. 2012; 22:210–5.
Article33. Ikuno Y, Nagai Y, Matsuda S, et al. Two-year visual results for older Asian women treated with photodynamic therapy or bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol. 2010; 149:140–6.
Article34. Peiretti E, Vinci M, Fossarello M. Intravitreal bevacizumab as a treatment for choroidal neovascularisation secondary to myopia: 4-year study results. Can J Ophthalmol. 2012; 47:28–33.
Article35. Axer-Siegel R, Ehrlich R, Weinberger D, et al. Photodynamic therapy of subfoveal choroidal neovascularization in high myopia in a clinical setting: visual outcome in relation to age at treatment. Am J Ophthalmol. 2004; 138:602–7.
Article36. Ikuno Y, Sayanagi K, Soga K, et al. Intravitreal Bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results. Am J Ophthalmol. 2009; 147:94–100.
Article37. Gharbiya M, Allievi F, Mazzeo L, Gabrieli CB. Intravitreal bevacizumab treatment for choroidal neovascularization in pathologic myopia: 12-month results. Am J Ophthalmol. 2009; 147:84–93.
Article38. Nakanishi H, Tsujikawa A, Yodoi Y, et al. Prognostic factors for visual outcomes 2-years after intravitreal bevacizumab for myopic choroidal neovascularization. Eye (Lond). 2011; 25:375–81.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term Therapeutic Effect of Intravitreal Bevacizumab (Avastin) on Myopic Choroidal Neovascularization
- Long-term Treatment Outcomes of Intravitreal Bevacizumab Treatment for Myopic Choroidal Neovascularization
- Short-term Efficacy of Intravitreal Ranibizumab for Myopic Choroidal Neovascularization
- Long-term Outcomes of Myopic Choroidal Neovascularization and Retinal Changes according to ATN Classification
- Clinical Effect of Intravitreal Bevacizumab Injection in Myopic Choroidal Neovascularization