J Korean Ophthalmol Soc.
2010 Mar;51(3):359-365.
Clinical Effect of Intravitreal Bevacizumab Injection in Myopic Choroidal Neovascularization
- Affiliations
-
- 1Department of Ophthalmology, School of Medicine, Pusan National University, Pusan, Korea. jlee@pusan.ac.kr
Abstract
- PURPOSE
To evaluate the therapeutic effect of bevacizumab in treating myopic choroidal neovascularization (CNV).
METHODS
Medical records of the eyes that underwent intravitreal bevacizumab injection for myopic CNV and were followed up for more than one year were reviewed retrospectively. Best corrected visual acuity (BCVA), foveal thickness in optical coherence tomography and fluorescein angiography were investigated.
RESULTS
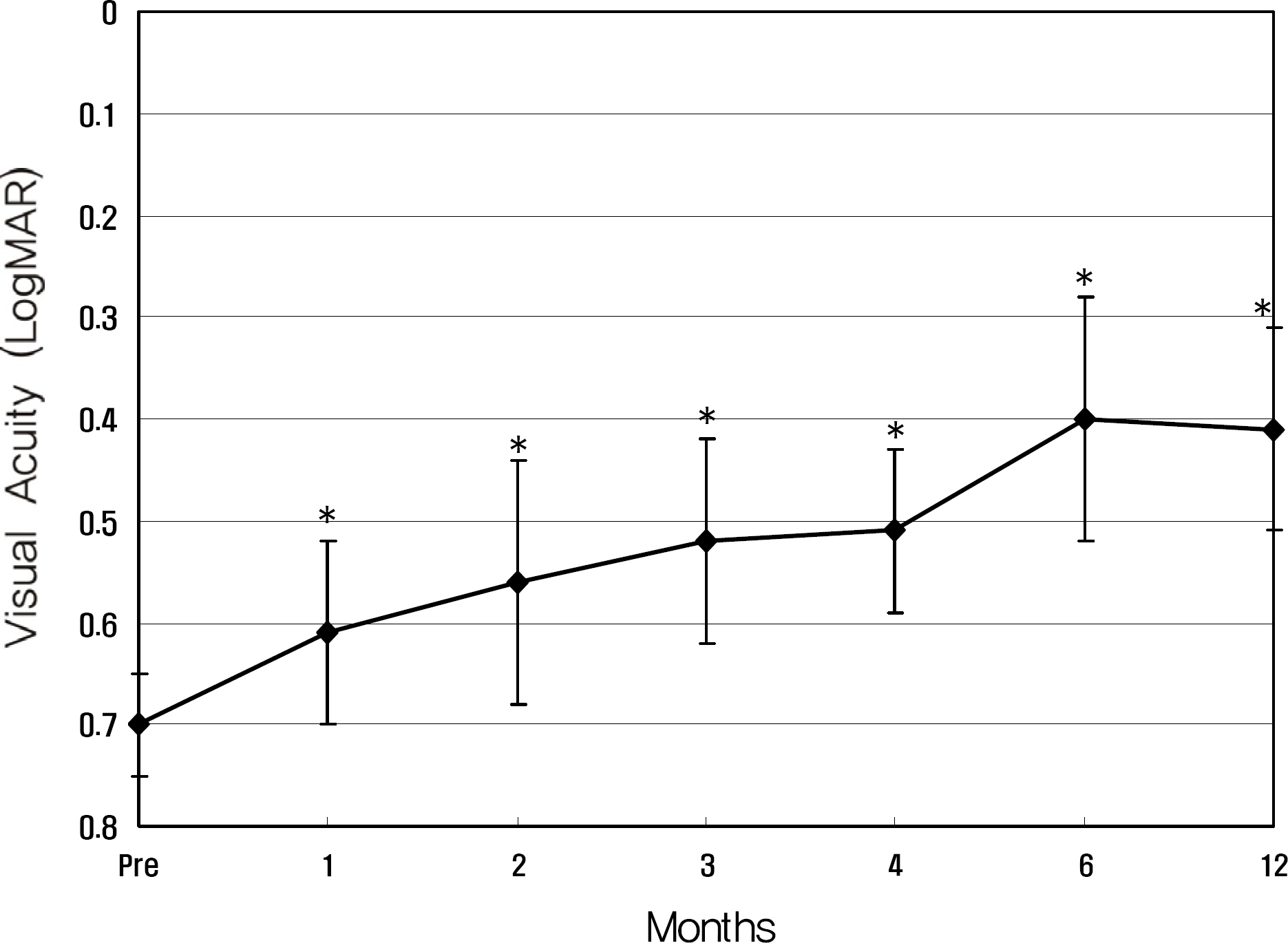
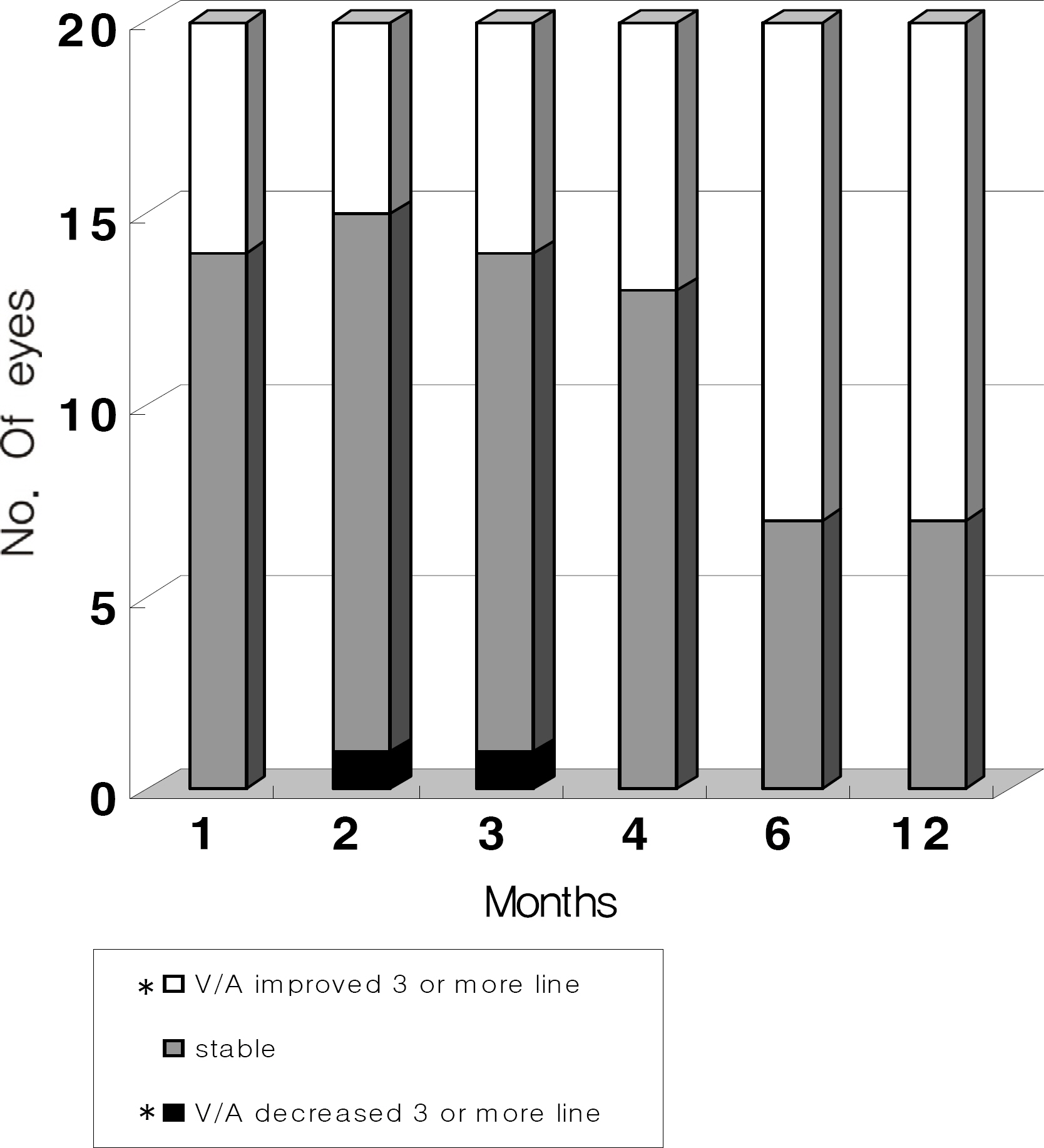
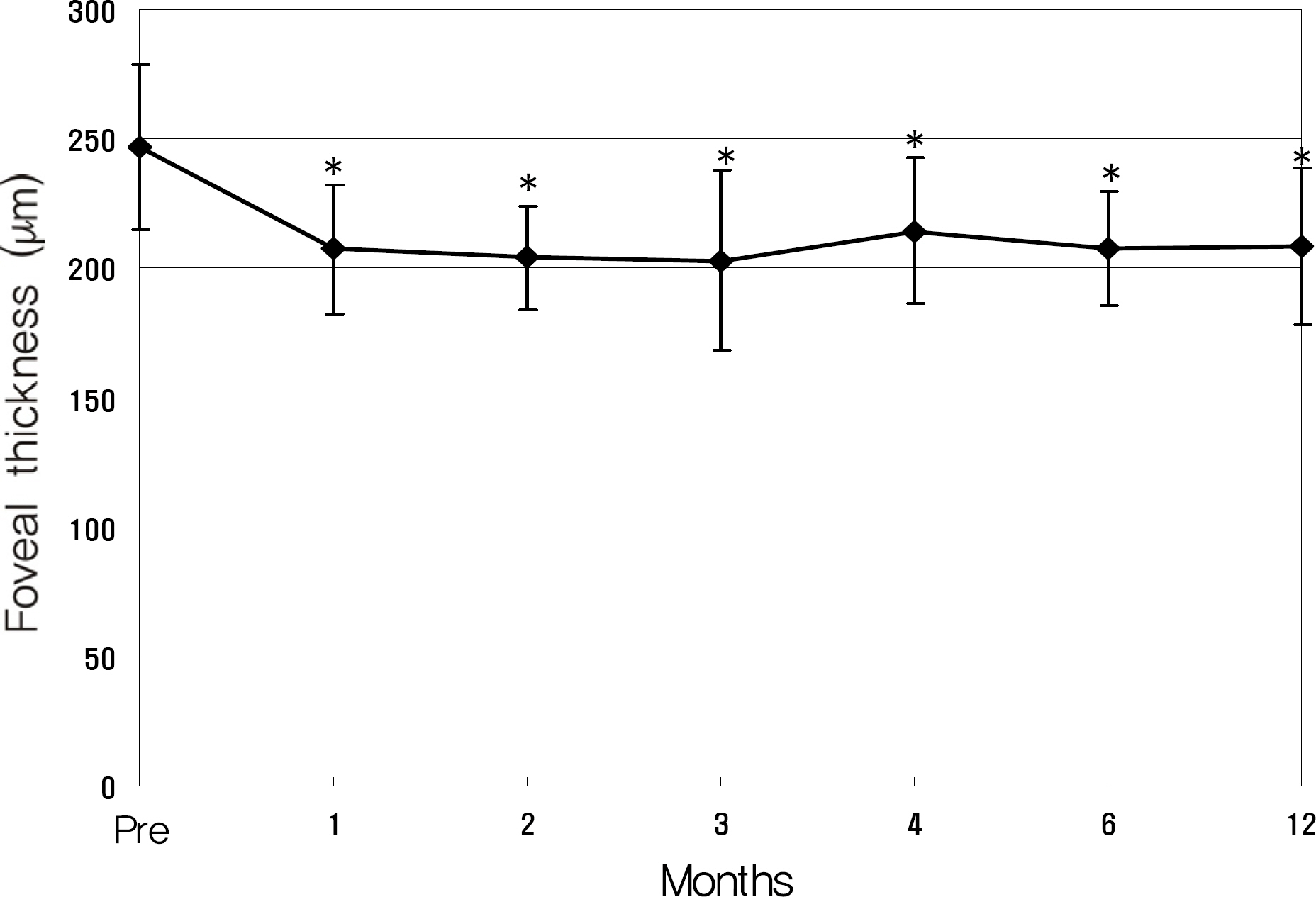
Twenty eyes of 18 patients were included in the present study. The average age was 41.5 years, average axial length was 28.5 mm, and average refractive error was -11.0 diopters. Visual acuity was maintained in all eyes, and 13 eyes improved by LogMAR 0.3 or more. Visual acuity improved significantly from 0.71 (0.2~2.0) to 0.40 (0.1~2.0, p=0.02) at six months and to 0.41 (0.1~2.0, p=0.03) at one year. Central foveal thickness significantly decreased from 247.0 micrometer to 207.5 micrometer (p=0.03) at six months and to 208.5 micrometer (p=0.04) at one year.
CONCLUSIONS
Anti-VEGF therapy using bevacizumab for the treatment of myopic CNV was effective in maintaining visual acuity.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Chung EJ, Oh HS, Koh HJ, Lee SC, Kwon OW. Photodynamic Therapy in Practice: A Review of Experiences with Myopic CNV in Korean Patients. J Korean Ophthalmol Soc. 2005; 46:664–70.2. Hotchkiss ML, Fine SL. Pathologic myopia and choroidal neovascularization. Am J Ophthalmol. 1981; 91:177–83.
Article3. Verteporfin in Photodynamic Therapy (VIP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin: 1year results of a randomized clinical trial-VIP report no. 1. Ophthalmology. 2001; 108:841–52.4. Verteporfin in Photodynamic Therapy(VIP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial-VIP report no. 3. Ophthalmology. 2003; 110:667–73.5. Kourlas H, Abrams P. Ranibizumab for the treatment of neovascular age-related macular degeneration: a review. Clinical Therapeutics. 2007; 29:1850–61.
Article6. Heier JS, Antoszyk AN, Pavan PR, et al. Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology. 2006; 113:633–4.7. Steidl SM, Pruett RC. Macular complications associated with posterior staphyloma. Am J Ophthalmol. 1997; 123:181–7.
Article8. Brancato R, Trabucchi G, Introini U, et al. Indocyanine green angiography (ICGA) in pathologic myopia. Eur J Ophthalmol. 1996; 6:39–43.9. Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal Neovascular lesions in age related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1991; 109:1220–31.10. Macular Photocoagulation Study Group. Subfoveal Neovascular lesions in age related macular degeneration: guidelines for evaluation and treatment in the macular photocoagulation study. Arch Ophthalmol. 1991; 109:1242–57.11. Macular Photocoagulation Study Group. Laser photocoagulation for juxtafoveal choroidal neovascularization: five-year results from randomized clinical trials. Arch Ophthalmol. 1994; 112:500–9.12. Macular Photocoagulation Study Group. Visual outcome after laser photocoagulation of subfoveal choroidal neovascularization secondary to age related macular degeneration. Arch Ophthalmol. 1994; 112:480–8.13. Cohen SY, Laroche A, Leguen Y, et al. Etiology of choroidal neovascularization in young patients. Ophthalmology. 1996; 103:1241–5.
Article14. Bottoni F, Perego E, Airaghi P, et al. Surgical removal of subfoveal choroidal neovascular membranes in high myopia. Graefes Arch Clin Exp Ophthalmol. 1999; 237:573–82.
Article15. Thomas MA, Dickinson JD, Melberg NS, et al. Visual results after surgical removal of subfoveal choroidal neovascular membranes. Ophthalmology. 1994; 101:1384–96.
Article16. Ruiz-Moreno JM, De-la-Verga C. Surgical removal of subfoveal choroidal neovascularization in highly myopic patients. Br J Ophthalmol. 2001; 85:1041–3.17. Pece A, Vadalà M, Isola V, Matranga D. Photodynamic therapy with Verteporfin for juxtafoveal choroidal neovascularization in pathologic myopia: A long-term follow-up study. Am J Ophthalmol. 2007; 143:449–54.
Article18. Kondo M, Ito Y, Miyata K, et al. Effect of axial length on laser spot size during photodynamic therapy: an experimental study in monkeys. Am J Ophthalmol. 2006; 141:214–5.
Article19. Yamamoto I, Rogers AH, Reichel E. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularization secondary to pathological myopia. Br J Ophthalmol. 2007; 91:157–60.20. Sakaguchi H, Ikuno Y, Gomi F, et al. Intravitreal injection of bevacizumab for choroidal neovascularization associated with pathological myopia. Br J Ophthalmol. 2007; 91:161–5.21. Song MH, Kim JY, Roh YJ. abdominal efficacy of intravitreal ranicizumab for myopic choroidal neovascularization. J Korean Ophthalmol Soc. 2009; 50:1027–34.22. Wai-Man Chan, Timothy Y, David T, Lam DS. Intravitreal bevacizumab(Avastin) for Myopic Choroidal Neovascularization. Ophthalmology. 2007; 114:2190–6.23. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranivizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:679–80.24. Ikuno Y, Sayanagi K, Soga K, et al. Intravitreal Bevacizumab for choroidal neovascularization attributable to pathological myopia: One-Year Results. Am J Ophthalmol. 2009; 147:94–101.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term Therapeutic Effect of Intravitreal Bevacizumab (Avastin) on Myopic Choroidal Neovascularization
- Effect of High-dose Intravitreal Bevacizumab Injection on Refractory Idiopathic Choroidal Neovasculariz
- A Case of Intravitreal Bevacizumab Injection for the Treatment of Choroidal Neovascularization in Morning Glory Syndrome
- Multifocal Electroretinogram Findings after Intravitreal Bevacizumab Injection in Choroidal Neovascularization of Age-Related Macular Degeneration
- Choroidal Neovascularization in a Patient with Best Disease