J Korean Ophthalmol Soc.
2011 Jan;52(1):34-40. 10.3341/jkos.2011.52.1.34.
Long-term Therapeutic Effect of Intravitreal Bevacizumab (Avastin) on Myopic Choroidal Neovascularization
- Affiliations
-
- 1Department of Ophthalmology, Dankook University Hospital, Cheonan, Korea. changmh@dankook.ac.kr
- KMID: 2214135
- DOI: http://doi.org/10.3341/jkos.2011.52.1.34
Abstract
- PURPOSE
To evaluate the long-term therapeutic effects of intravitreal bevacizumab on myopic choroidal neovascularization (CNV).
METHODS
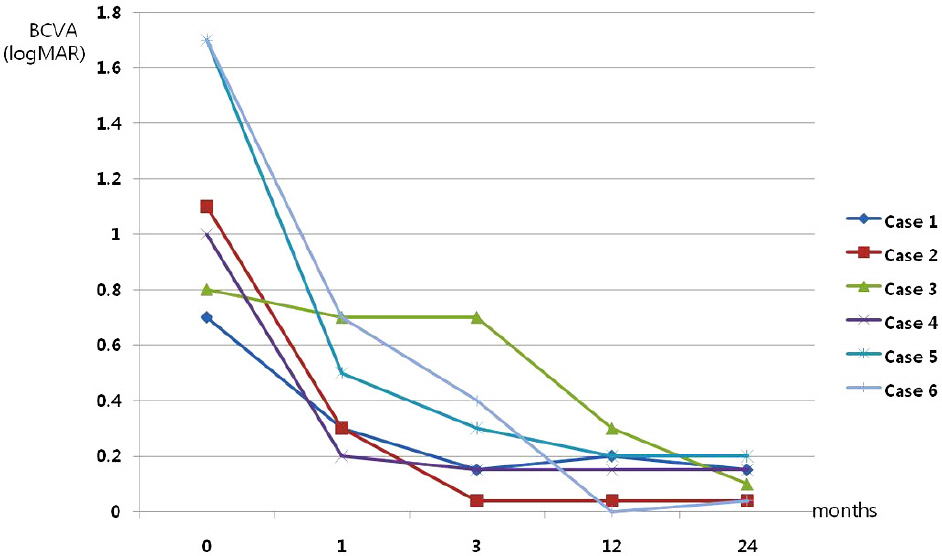
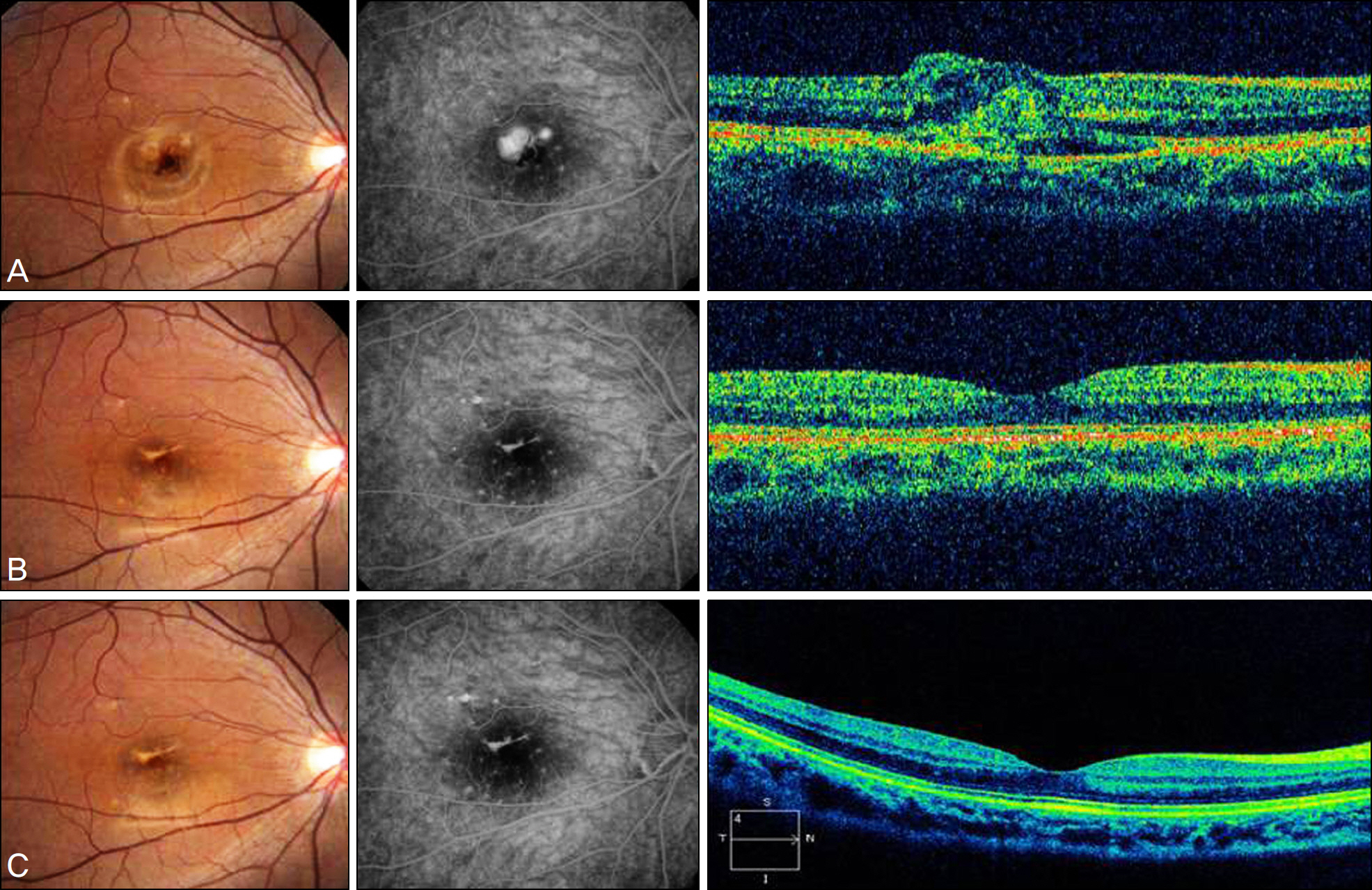
Medical records of 6 patients who underwent intravitreal bevacizumab injection for myopic CNV and were followed for more than 2 years, were retrospectively investigated. The best corrected visual acuity was compared at 1,3,12, and 24 months after injection. Two years after the injection, a fluorescein angiography and optical coherence tomography (OCT) were performed to evaluate the central macular thickness and leakage of CNV.
RESULTS
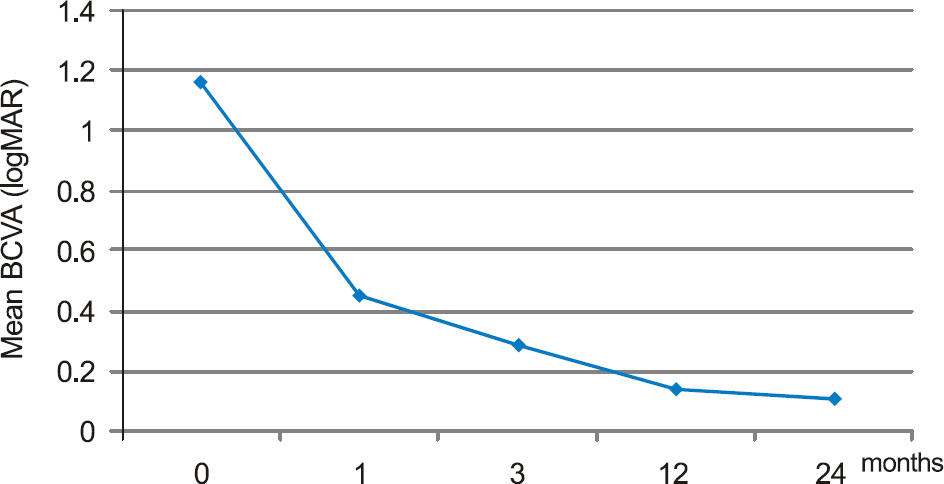
The mean best corrected visual acuity was 1.16 +/- 0.43 (logMAR), 0.45 +/- 0.21 (logMAR), 0.29 +/- 0.23 (logMAR), 0.14 +/- 0.11 (logMAR), and 0.11 +/- 0.06 (logMAR) at baseline, 1, 3, 12, and 24 months after injection, respectively.
CONCLUSIONS
Intravitreal bevacizumab injection for the treatment of myopic CNV was effective in maintaining postoperative visual acuity for 2 years.
MeSH Terms
Figure
Reference
-
References
1. Noble KG, Carr RE. Pathologic myopia. Ophthalmology. 1982; 89:1099–100.
Article2. Curtin BJ. Physiologic vs pathologic myopia: genetic vs aberrations. Ophthalmology. 1979; 86:681–91.3. Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina. 1992; 12:127–33.
Article4. Hayasaka S, Uchida M, Setogawa T. Subretinal hemorrhages with or without choroidal neovascularization in the maculas of patients with pathologic myopia. Graefes Arch Clin Exp Ophthalmol. 1990; 228:277–80.
Article5. Hotchkiss ML, Fine SL. Pathologic myopia and choroidal aberrations. Am J Ophthalmol. 1981; 91:177–83.6. Levy JH, Pollock HM, Curtin BJ. The Fuchs' spot: an ophthalmoscopic and fluorescein angiographic study. Ann Ophthalmol. 1977; 9:1433–42.7. Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology. 2003; 110:1297–305.8. McCarty CA, Livingston PM, Taylor HR. Prevalence of myopia in adults: implications for refractive surgeons. J Refract Surg. 1997; 13:229–34.
Article9. Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol. 2003; 87:570–3.
Article10. Chan WM, Ohji M, Lai TY, et al. Choroidal neovascularisation in pathological myopia: an update in management. Br J Ophthalmol. 2005; 89:1522–8.
Article11. Secretan M, Kuhn D, Soubrane G, et al. Long-term visual outcome of choroidal neovascularization in pathologic myopia: natural history and laser. Eur J Ophthalmol. 1997; 7:307–16.12. Steidl SM, Pruett RC. Macular complications associated with posterior staphyloma. Am J Ophthalmol. 1997; 123:181–7.
Article13. Ruiz-Moreno JM, Montero JA. Long-term visual acuity after argon green laser photocoagulation of juxtafoveal choroidal neovascularization in highly myopic eyes. Eur J Ophthalmol. 2002; 12:117–22.
Article14. Soubrane G. Choroidal neovascularization in pathologic myopia: recent developments in diagnosis and treatment. Surv Ophthalmol. 2008; 53:121–38.
Article15. Ruiz-Moreno JM, de la Vega C. Surgical removal of subfoveal choroidal neovascularisation in highly myopic patients. Br J Ophthalmol. 2001; 85:1041–3.
Article16. Verteporfin in Photodynamic Therapy (VIP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin 1-year results of a randomized clinical trial VIP report No. 1. Ophthalmology. 2001; 108:841–52.17. Blinder KJ, Blumenkranz MS, Bressler NM, et al. aberrations therapy of subfoveal choroidal neovascularization in pathologic myopia 2-year results of a randomized clinical trial VIP Report No. 3. Ophthalmology. 2003; 110:667–73.18. Chan WM, Lai TY, Kiu DT, Lam DS. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization six-month results of a prospective pilot study. Ophthalmology. 2007; 114:2190–6.19. Kim KH, Jung JH, Lee JE, Oum BS. Clinical effect of intravitreal bevacizumab injection in myopic choroidal neovascularization. J Korean Ophthalmol Soc. 2010; 51:359–65.
Article20. Gharbiya M, Allievi F, Mazzeo L, Gabrieli CB. Intravitreal bevacizumab treatment for choroidal neovascularization in pathologic myopia: 12-month results. Am J Ophthalmol. 2009; 147:84–93.
Article21. Ikuno Y, Sayanagi K, Soga K, et al. Intravitreal bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results. Am J Ophthalmol. 2009; 147:94–100.
Article22. Hampton GR, Kohen D, Bird AC. Visual prognosis of disciform degeneration in myopia. Ophthalmology. 1983; 90:923–6.
Article23. Hayashi K, Ohno-Matsui , Yoshida T. Characteristics of patients with a favorable natural course of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2005; 243:13–9.
Article24. Avila MP, Weiter JJ, Jalkh AE, et al. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology. 1984; 91:1573–81.
Article25. Fried M, Siebert A, Meyer-Schwickerath G. A natural history of Fuchs' spot: a long-term follow-up study. Doc Ophthalmol. 1981; 28:215–21.26. Tabandeh H, Flynn HW Jr, Scott IU, et al. Visual acuity outcomes of patients 50 years of age and older with high myopia and untreated choroidal neovascularization. Ophthalmology. 1999; 106:2063–7.
Article27. Bottoni F, Perego E, Airaghi P, et al. Surgical removal of subfoveal choroidal neovascular membranes in high myopia. Graefes Arch Clin Exp Ophthalmol. 1999; 237:573–82.
Article28. Uemura A, Thomas MA. Subretinal surgery for choroidal neovascularization in patients with high myopia. Arch Ophthalmol. 2000; 118:344–50.
Article29. Yamamoto I, Rogers AH, Reichel E, et al. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularization secondary to pathologic myopia. Br J Ophthalmol. 2007; 91:157–60.30. Hayashi K, Ohno-Matsui K, Teramukai S, et al. Comparison of visual outcome and regression pattern of myopic choroidal neovascularization after intravitreal bevacizumab or after photodynamic therapy. Am J Ophthalmol. 2009; 148:396–408.
Article31. Ruiz-Moreno JM, Montero JA. Intravitreal bevacizumab to treat myopic choroidal neovascularization: 2-year outcome. Graefes Arch Clin Exp Ophthalmol. 2010; 248:937–41.
Article32. Ikuno Y, Nagai Y, Matsuda S, et al. Two-year visual results for older asian women treated with photodynamic therapy or aberrations for myopic choroidal neovascularization. Am J Ophthalmol. 2010; 149:140–6.33. Kakinoki M, Sawada O, Sawada T, et al. Comparison of macular thickness between cirrus HD-OCT and stratus OCT. Ophthalmic Surg Lasers Imaging. 2009; 40:135–40.
Article34. Leung CK, Cheung CY, Weinreb RN, et al. Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2008; 49:4893–7.
Article35. Moon SW, Kim ES, Kim YG, et al. The comparison of macular thickness measurements and repeatabilities between time domain and spectral domain OCT. J Korean Ophthalmol Soc. 2009; 50:1050–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term Effect of Intravitreal Bevacizumab for CNV Secondary to Age-Related Macular Degeneration
- Long-term Treatment Outcomes of Intravitreal Bevacizumab Treatment for Myopic Choroidal Neovascularization
- Effect of High-dose Intravitreal Bevacizumab Injection on Refractory Idiopathic Choroidal Neovasculariz
- Clinical Effect of Intravitreal Bevacizumab Injection in Myopic Choroidal Neovascularization
- Risk Factors for Aggravation of Myopic Retinoschisis after Intravitreal Bevacizumab Injection in Myopic Choroidal Neovascularization