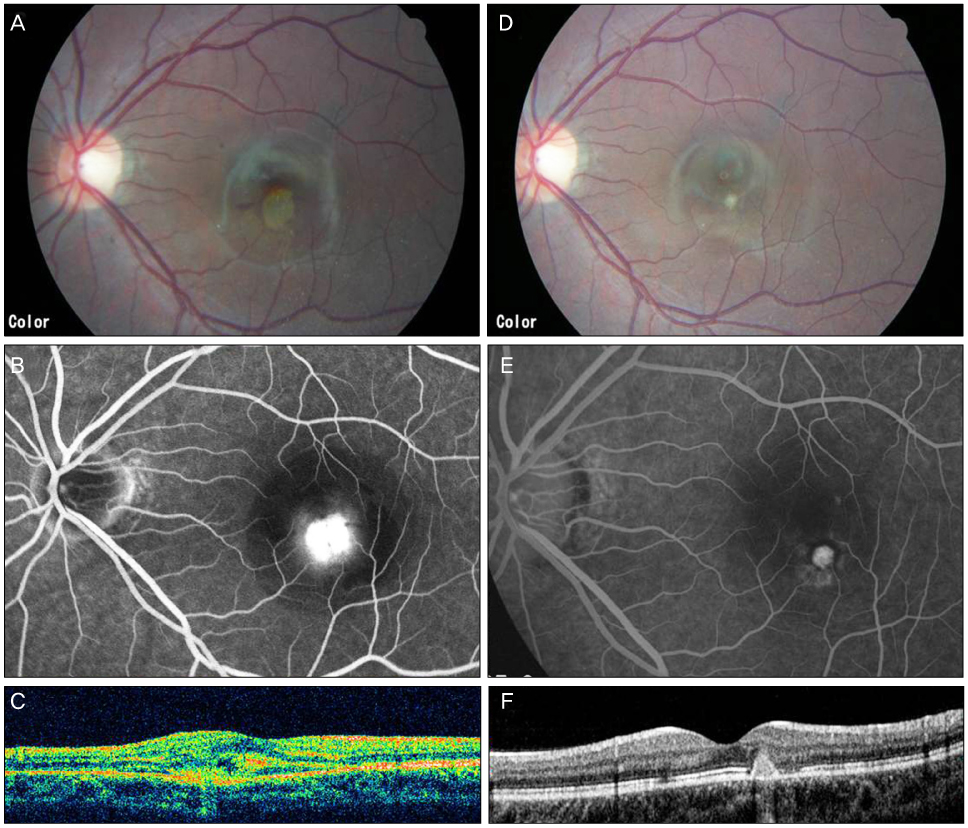
J Korean Ophthalmol Soc.
2012 Dec;53(12):1807-1813. 10.3341/jkos.2012.53.12.1807.
Comparison of Two Doses of IVB and Prognostic Factor on Myopic CNV : 1-Year Outcome
- Affiliations
-
- 1Myung-Gok Eye Research Institute, Department of Ophthalmology, Kim's Eye Hospital, Konyang University, Seoul, Korea. yjlew@kimeye.com
- KMID: 2216395
- DOI: http://doi.org/10.3341/jkos.2012.53.12.1807
Abstract
- PURPOSE
To compare the efficacy of 3 consecutive monthly intravitreal bevacizumab (IVB) injections (fixed regimen group) with a single IVB injection (PRN group) on patients with myopic choroidal neovascularization (CNV) and to determine the prognostic factors associated with IVB injection outcomes.
METHODS
23 Twenty-three eyes in 21 patients with myopic CNV (14 eyes in the fixed regimen group and 9 eyes in the PRN group) treated with IVB were studied retrospectively. Best corrected visual acuity (BCVA), central macular thickness (CMT), the size of CNV prior to the initial IVB injection, CMT at the completion of treatment, and the number of IVB injections during the study period was measured.
RESULTS
IVB resulted in improved BCVA and decreased CMT in both groups, and the differences before and after IVB injections were significantly correlated. Average injection time in the fixed regimen group and PRN group was 3.4 +/- 0.9 and 1.5 +/- 0.7 respectively, and was not statistically significant (p = 0.16). Differences between the groups in BCVA (p = 0.83) and CMT (p = 0.38) were not significantly correlated. Among the variables measured prior to IVB injection that affected final BCVA was age (p = 0.01).
CONCLUSIONS
In the present study, a single injection of IVB compared to 3 consecutive IVB injections in patients with myopic CNV resulted in similar outcomes. In the future, these results can be considered as a reference when designing treatments for myopic CNV patients.
MeSH Terms
Figure
Reference
-
1. Cohen SY, Laroche A, Leguen Y, et al. Etiology of choroidal neovascularization in young patients. Ophthalmology. 1996. 103:1241–1244.2. Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology. 2003. 110:1297–1305.3. McCarty CA, Livingston PM, Taylor HR. Prevalence of myopia in adults: implications for refractive surgeons. J Refract Surg. 1997. 13:229–234.4. Blinder KJ, Blumenkranz MS, Bressler NM, et al. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial--VIP report no. 3. Ophthalmology. 2003. 110:667–673.5. Brancato R, Pece A, Avanza P, Radrizzani E. Photocoagulation scar expansion after laser therapy for choroidal neovascularization in degenerative myopia. Retina. 1990. 10:239–243.6. Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006. 26:383–390.7. Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006. 26:495–511.8. Bashshur ZF, Bazarbachi A, Schakal A, et al. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006. 142:1–9.9. Gharbiya M, Allievi F, Mazzeo L, Gabrieli CB. Intravitreal bevacizumab treatment for choroidal neovascularization in pathologic myopia: 12-month results. Am J Ophthalmol. 2009. 147:84–93.e1.10. Ikuno Y, Sayanagi K, Soga K, et al. Intravitreal bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results. Am J Ophthalmol. 2009. 147:94–100.e1.11. Kim KH, Jung JH, Lee JE, Oum BS. Clinical effect of intravitreal bevacizumab injection in myopic choroidal neovascularization. J Korean Ophthalmol Soc. 2010. 51:359–365.12. Forte R, Cennamo GL, Finelli ML, de Crecchio G. Comparison of time domain Stratus OCT and spectral domain SLO/OCT for assessment of macular thickness and volume. Eye (Lond). 2009. 23:2071–2078.13. Ruiz-Moreno JM, Montero JA. Long-term visual acuity after argon green laser photocoagulation of juxtafoveal choroidal neovascularization in highly myopic eyes. Eur J Ophthalmol. 2002. 12:117–122.14. Vip Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin: 1-year results of a randomized clinical trial-VIP report no. 1. Ophthalmology. 2001. 108:841–852.15. Chung EJ, Oh HS, Koh HJ, et al. Photodynamic therapy in practice: A review of experiences with myopic CNV in Korean patients. J Korean Ophthalmol Soc. 2005. 46:664–670.16. Ruiz-Moreno JM, de la Vega C. Surgical removal of subfoveal choroidal neovascularisation in highly myopic patients. Br J Ophthalmol. 2001. 85:1041–1043.17. Uemura A, Thomas MA. Subretinal surgery for choroidal neovascularization in patients with high myopia. Arch Ophthalmol. 2000. 118:344–350.18. Bottoni F, Perego E, Airaghi P, et al. Surgical removal of subfoveal choroidal neovascular membranes in high myopia. Graefes Arch Clin Exp Ophthalmol. 1999. 237:573–582.19. Ruiz-Moreno JM, Montero JA. Intravitreal bevacizumab to treat myopic choroidal neovascularization: 2-year outcome. Graefes Arch Clin Exp Ophthalmol. 2010. 248:937–941.20. Ruiz-Moreno JM, Montero JA, Amat-Peral P. Myopic choroidal neovascularization treated by intravitreal bevacizumab: comparison of two different initial doses. Graefes Arch Clin Exp Ophthalmol. 2011. 249:595–599.21. Sakaguchi H, Ikuno Y, Gomi F, et al. Intravitreal injection of bevacizumab for choroidal neovascularisation associated with pathological myopia. Br J Ophthalmol. 2007. 91:161–165.22. Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularisation: 1-year results of a prospective pilot study. Br J Ophthalmol. 2009. 93:150–154.23. Wu PC, Chen YJ. Intravitreal injection of bevacizumab for myopic choroidal neovascularization: 1-year follow-up. Eye (Lond). 2009. 23:2042–2045.24. Seo YS, Chang MH. Long-term Therapeutic effect of Intravitreal Bevacizumab (Avastin) on Myopic Choroidal Neovascularization. J Korean Ophthalmol Soc. 2011. 52:34–40.25. Hayashi K, Ohno-Matsui K, Yoshida T. Characteristics of patients with a favorable natural course of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2005. 243:13–19.26. Nakanishi H, Tsujikawa A, Yodoi Y, et al. Prognostic factors for visual outcomes 2-years after intravitreal bevacizumab for myopic choroidal neovascularization. Eye (Lond). 2011. 25:375–381.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Relapsed Inflammatory Choroidal Neovascularization in Punctate Inner Choroidopathy after Bevacizumab
- Preferential Hyperacuity Perimeter (PHP) in Myopic CNV
- Indocyanine Green Angiographic Features of Myopic Subfoveal Choroidal Neovascularization as a Prognostic Factor after Photodynamic Therapy
- Long-term Therapeutic Effect of Intravitreal Bevacizumab (Avastin) on Myopic Choroidal Neovascularization
- Bevacizumab Monotherapy Versus Combined Therapy with Photodynamic Therapy for Occult Choroidal Neovascularization in Age-Related Macular Degeneration