Korean J Ophthalmol.
2006 Mar;20(1):18-25. 10.3341/kjo.2006.20.1.18.
Indocyanine Green Angiographic Features of Myopic Subfoveal Choroidal Neovascularization as a Prognostic Factor after Photodynamic Therapy
- Affiliations
-
- 1Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. hjkoh@yumc.yonsei.ac.kr
- 2Department of Ophthalmology, Youndong Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1099068
- DOI: http://doi.org/10.3341/kjo.2006.20.1.18
Abstract
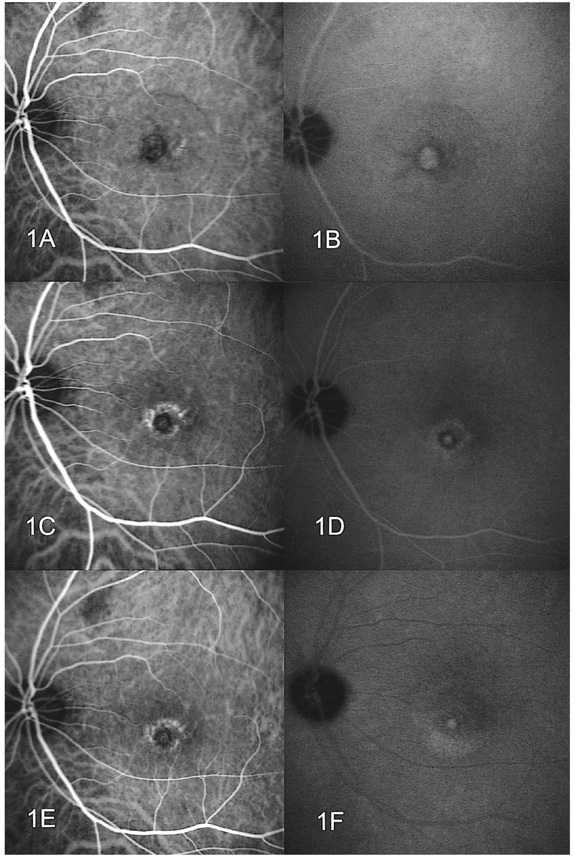
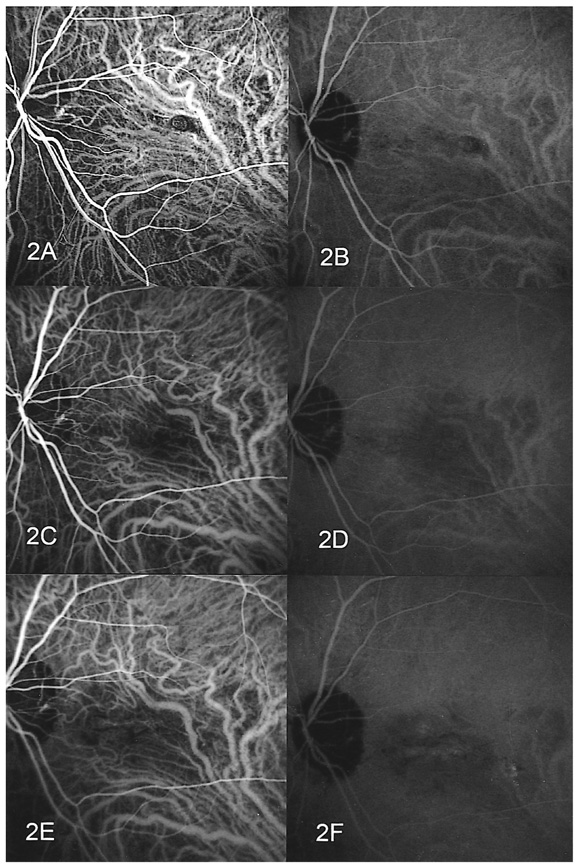
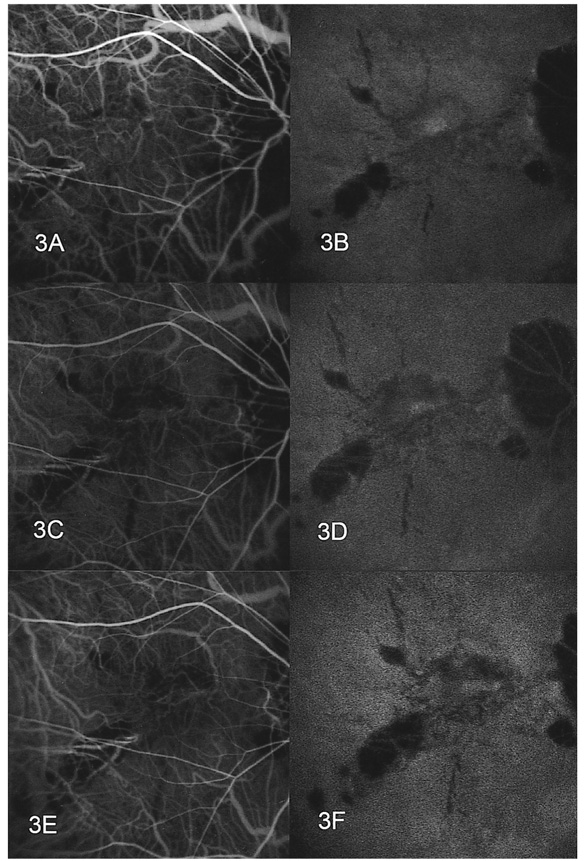
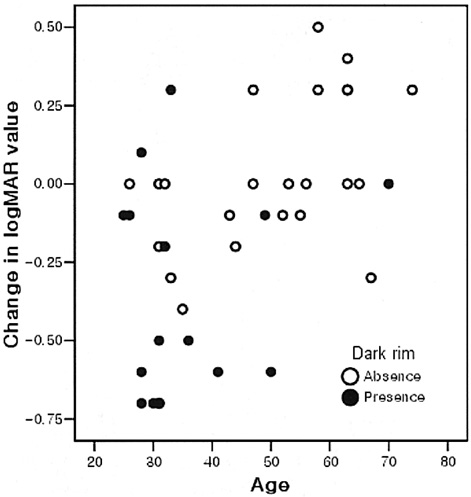
- PURPOSE: To determine the influence of clinical features and Indocyanine green (ICG) angiographic features on the visual outcome of patients with myopic sub-foveal choroidal neovascularization (CNV) who received photodynamic therapy (PDT). METHODS: Thirty-six consecutive patients (39 eyes) with myopic CNV who were followed up for more than one year after PDT were enrolled in this study. Clinical features included age, gender, refractive error, great linear dimension, and subretinal hemorrhage. ICG features included the lesion size, lacquer cracks, hypofluorescence surrounding the CNV (dark rim), peripapillary atrophy size, and visible prominent choroidal veins under the macula. Linear regression analysis was performed using the change in visual acuity (delta logMAR) as the dependent variable and the above factors as independent variables. RESULTS: At one-year follow-up after PDT, a younger age (p=0.002) and the presence of a dark rim (p=0.002) were significantly correlated with an improvement of visual acuity (decrement in logMAR) after PDT. Other factors had no significant influence on changes in visual acuity. CONCLUSIONS: Younger patients and patients with a dark rim on ICG angiography had a higher chance of visual improvement after PDT in myopic CNV.
Keyword
MeSH Terms
-
Visual Acuity
Retrospective Studies
Prognosis
*Photochemotherapy
Myopia/*complications/pathology
Middle Aged
Male
Indocyanine Green/*diagnostic use
Humans
Fundus Oculi
Fovea Centralis/*pathology
Follow-Up Studies
Fluorescein Angiography/*methods
Female
Coloring Agents/*diagnostic use
Choroidal Neovascularization/complications/drug therapy/*pathology
Aged
Adult
Figure
Cited by 1 articles
-
Predictive Factors for Visual Outcome after Treatment for Myopic Choroidal Neovascularization
Ha Na Oh, Joo Eun Lee, Hyun Woong Kim, Il Han Yun
J Korean Ophthalmol Soc. 2013;54(4):610-617. doi: 10.3341/jkos.2013.54.4.610.
Reference
-
1. Ghafour IM, Allan D, Foulds WS. Common causes of blindness and visual handicap in the west of Scotland. Br J Ophthalmol. 1986. 67:209–213.2. Sperduto RD, Seigel D, Roberts J, et al. Prevalence of myopia in the Unites States. Arch Ophthalmol. 1983. 101:405–407.3. Curtin BJ, Karlin DB. Axial length measurements and fundus changes in the myopic eye. Am J Ophthalmol. 1971. 71:42–50.4. Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina. 1992. 12:127–133.5. Levy JH, Pollock HM, Curtin BJ. The Fuchs' spot: an opthalmoscopic and fluorescein angiographic study. Ann Ophthalmol. 1977. 9:1433–1443.6. Verteporfin in Photodynamic Therapy (VIP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin: 1-year results of a randomized clinical trial - VIP report No. 1. Ophthalmology. 2001. 108:841–852.7. Verteporfin in Photodynamic Therapy (VIP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin: 2-year results of a randomized clinical trial - VIP report No. 3. Ophthalmology. 2003. 110:667–673.8. Montero JA, Ruiz-Moreno JM. Verteporfin photodynamic therapy in highly myopic subfoveal choroidal neovascularization. Br J Ophthalmol. 2003. 87:173–176.9. Ergun E, Heinzl H, Stur M. Prognostic factors influencing visual outcome of photodynamic therapy for subfoveal choroidal neovascularization in Pathologic myopia. Am J Ophthalmol. 2004. 138:434–438.10. Axer-Siegel R, Ehrlich R, Weinberger D, et al. Photodynamic therapy of subfoveal choroidal neovascularization in high myopia in a clinical setting: visual outcome in relation to age at treatment. Am J Ophthalmol. 2004. 138:602–607.11. Avetisov ES, Salvitskaya NF. Some features of ocular microcirculation in myopia. Ann Ophthalmol. 1977. 9:1261–1264.12. Curtin BJ. The pathogenesis of congenital myopia: a study of 66 cases. Arch Ophthalmol. 1963. 6:166–173.13. Noble KG, Carr RE. Pathologic myopia. Ophthalmology. 1982. 89:1099–1100.14. Steidl SM, Pruett RC. Macular complications associated with posterior staphyloma. Am J Ophthalmol. 1997. 123:181–187.15. Scheider A, Kaboth A, Neuhauser L. Detection of subretinal neovascular membranes with Indocyanine green and an infrared scanning laser ophthalmoscope. Am J Ophthalmol. 1992. 113:45–51.16. Iida T, Hagimura N, Kishi S, Shimizu K. Indocyanine green angiographic features of idiopathic submacular choroidal neovascularization. Am J Ophthalmol. 1998. 126:70–76.17. Quaranta M, Arnold J, Coscas G, et al. Indocyanine green angiographic features of pathologic myopia. Am J Ophthalmol. 1996. 122:663–671.18. Fukushima I, Takahashi K, Nishimura T, et al. Dark rim around choroidal neovascularization in indocyanine green angiography. Nippon Ganka Gakkai Zasshi. 1995. 99:1262–1270.19. Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin. One year results of 2 randomized clinical trials - TAP Report No. 1. Arch Opthalmol. 1999. 117:1329–1345.20. Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in agerelated macular degeneration with verteporfin. Two year results of 2 randomized clinical trials - TAP Report No. 2. Arch Opthalmol. 2001. 119:198–207.21. Parodi MB, Pozzo SD, Ravalico G. Angiographic features after photodynamic therapy for choroidal neovascularization in age related macular degeneration and pathologic myopia. Br J Ophthalmol. 2003. 87:177–183.22. Axer-Siegel RD, Cotlear D, Priel E, et al. Indocyanine green angiography in high myopia. Ophthalmic Surg Lasers Imaging. 2004. 35:139–145.23. Yannuzzi LA, Flower RW, Slakter JS. Indocyanine green angiography. 1997. 1st ed. St. Louis: Mosby;chap. 85.24. Tokoro T, Mochizuki M, Yasuzumi K, et al. Peripapillary crescent enlargement in highly myopic eyes evaluated by fluorescein and indocyanine green angiography. Br J Ophthalmol. 2003. 87:1088–1090.25. Lam DSC, Chan W-M, Liu DTL, et al. Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization of pathologic myopia in Chinese eyes: a prospective series of 1 and 2 year follow up. Br J Ophthalmol. 2004. 88:1315–1319.26. Fried M, Siebert A, Meyer-Schwickerath G. Natural history of Fuchs' spot: a long-term follow up study. Doc Ophthalmol. 1981. 28:215–221.27. Avila MP, Weiter JJ, Jalkh AE, et al. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology. 1984. 91:1573–1581.28. Yoshida T, Ohno-Matsui K, Ohtake Y, et al. Long-term visual prognosis of choroidal neovascularization in high myopia. Ophthalmology. 2002. 109:712–719.29. Hayashi K, Ohno-Matsui K, Yoshida T, et al. Characteristics of patients with favorable natural course of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2005. 243:13–19.30. Hotchkiss ML, Fine SL. Pathologic myopia and choroidal neovascularization. Am J Ophthalmol. 1981. 91:177–183.31. Hampton GR, Kohen D, Bird AC. Visual prognosis of disciform degeneration in myopia. Ophthalmology. 1983. 90:923–926.32. Schmidt-Erfurth U, Michels S, Barbazetto I, Laqua H. Photodynamic effects on choroidal neovascularization and physiologic choroid. Invest Ophthalmol Vis Sci. 2002. 43:830–841.33. Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularization in pathologic myopia. Br J Ophthalmol. 2003. 87:570–573.34. Bischoff PM, Flower RW. High blood pressure in choroidal arteries as possible pathogenic mechanism in senile macular degeneration. Am J Ophthalmol. 1983. 96:398–399.35. Dimitrova G, Tamaki Y, Kato S, Nagahara M. Retrobulbar circulation in myopic patients with or without myopic choroidal neovascularization. Br J Ophthalmol. 2002. 86:771–773.36. Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic choroidal neovascularization. Ophthalmology. 2003. 110:1297–1305.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Subfoveal Choroidal Neovascularization following Multiple Evanescent White-Dot Syndrome
- The Evaluation of Photodynamic Therapy for Idiopathic Subfoveal Choroidal Neovascularization with Imaging Studies
- Photodynamic Therapy For Recurred Subfoveal Choroidal Neovascularization After Argon-Laser Photocoagulation
- Changes of Choroidal Perfusion in Indocyanine Green Angiography After Photodynamic Therapy for Choroidal Neovascularization
- Availability of Optical Coherence Tomography in Diagnosis and Classification of Choroidal Neovascularization