J Korean Ophthalmol Soc.
2015 Jan;56(1):99-103. 10.3341/jkos.2015.56.1.99.
Transcriptional Analysis of Nod-Like Receptors in a Mouse Model of Experimental Autoimmune Uveitis
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. jooyounoh77@gmail.com
- 2Department of Ophthalmology, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea.
- 3Laboratory of Ocular Regenerative Medicine and Immunology, Seoul Artificial Eye Center, Seoul National University Hospital Biomedical Research Institute, Seoul, Korea.
- KMID: 2216160
- DOI: http://doi.org/10.3341/jkos.2015.56.1.99
Abstract
- PURPOSE
To evaluate the transcription pattern of Nod-like receptors (NLRs), the intracellular sensors, to detect danger signals in murine eyes with experimental autoimmune uveitis (EAU).
METHODS
EAU was induced in B6 (C57BL/6) mice by subcutaneous injection of human interphotoreceptor retinoid binding protein and intraperitoneal injection of pertussis toxin. At 1, 2, and 3 weeks post-immunization, the eyeballs were extracted and subjected to histological and molecular assays using real-time reverse transcription polymerase chain reaction.
RESULTS
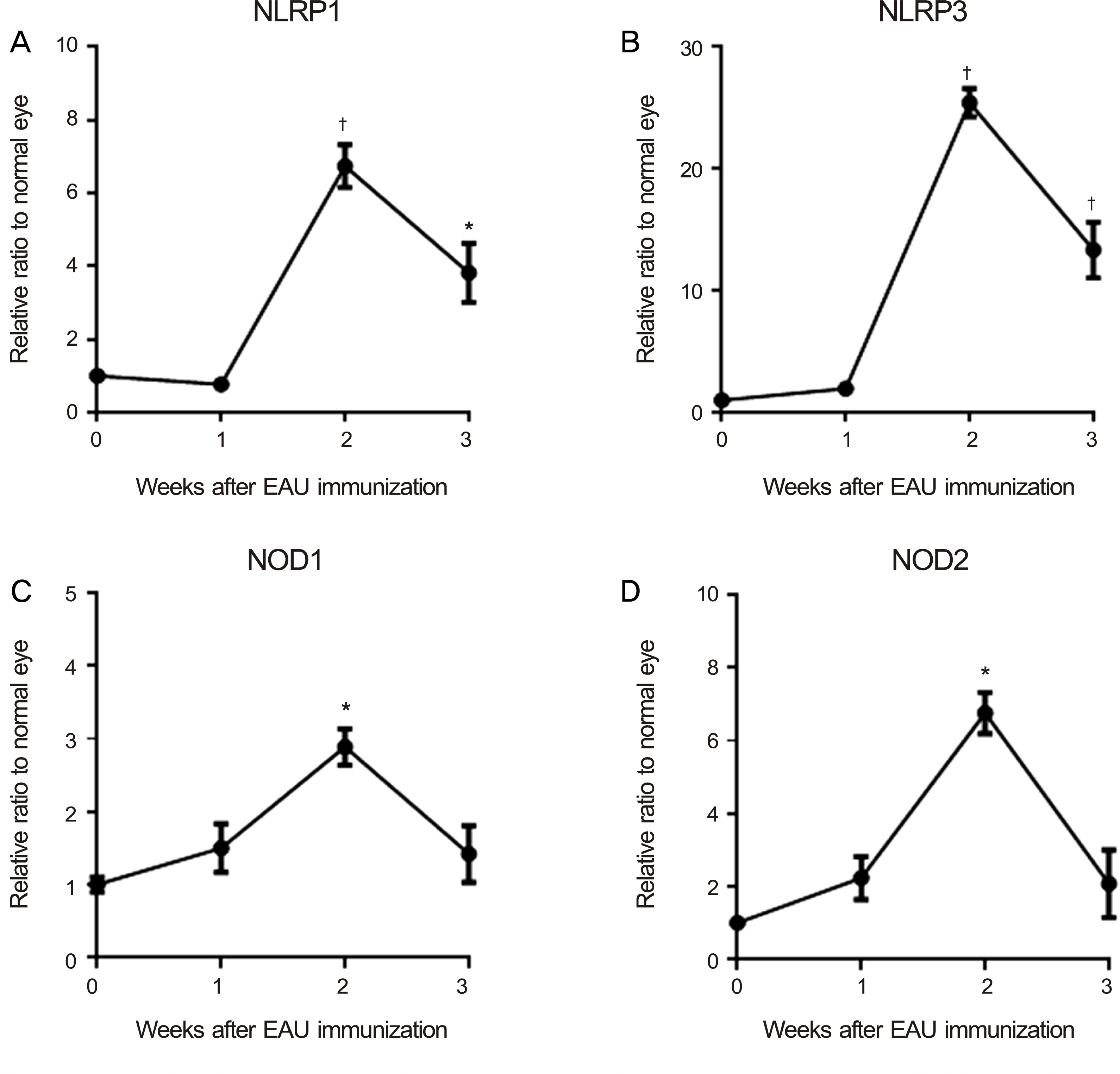
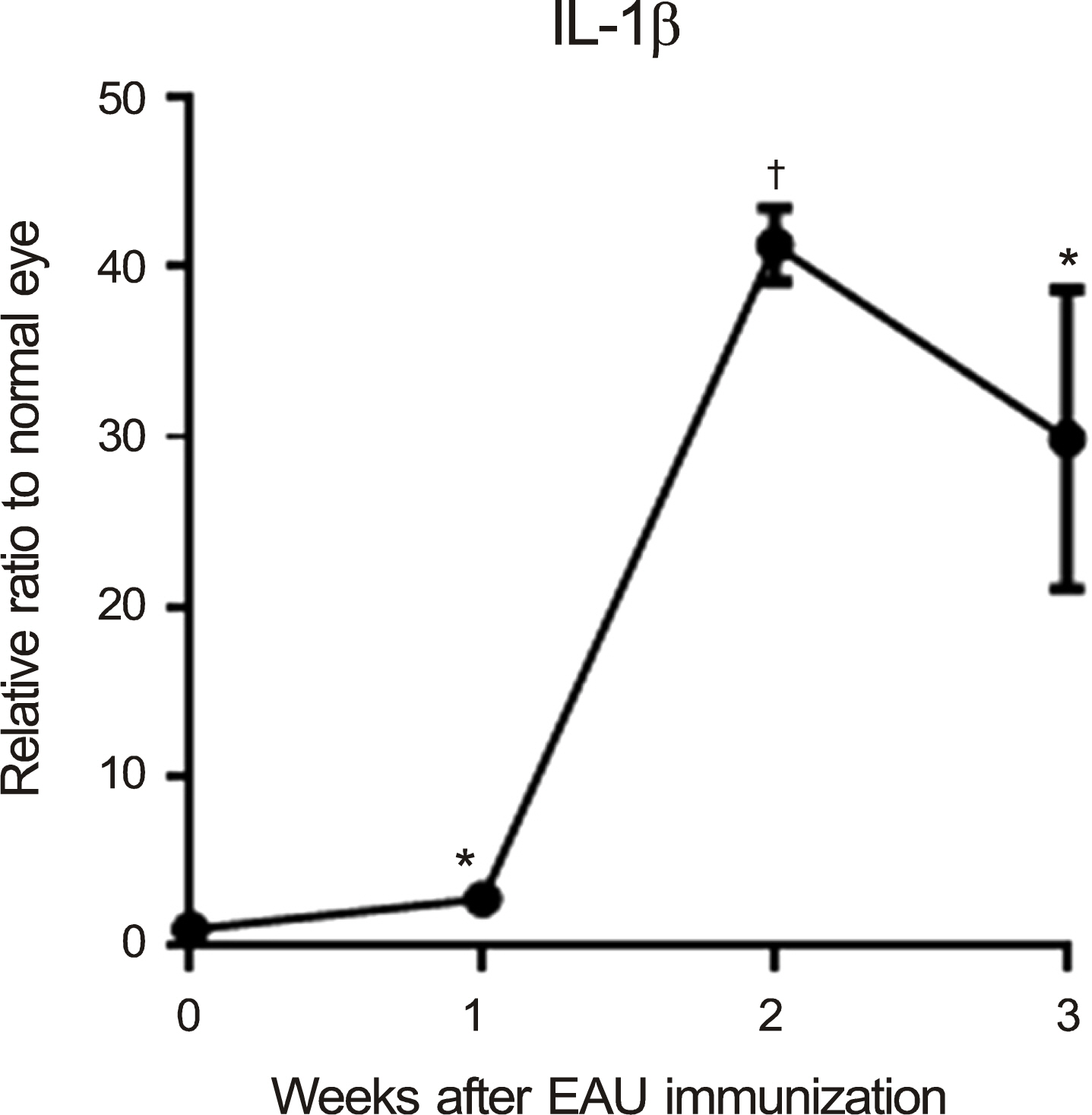
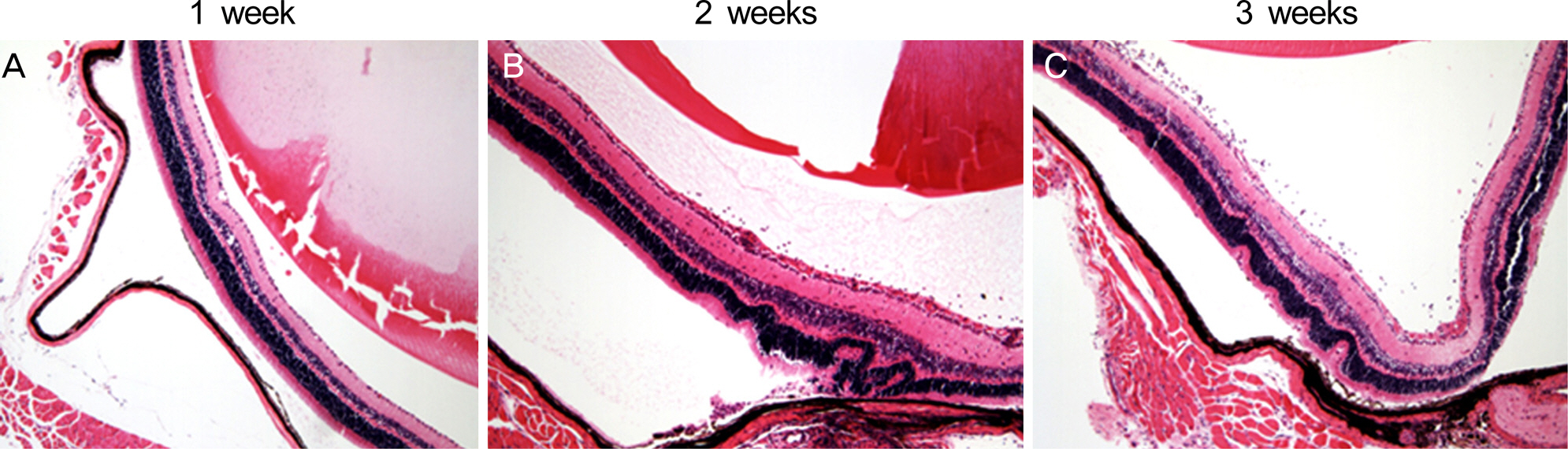
The levels of nucleotide-binding oligomerization domain, Leucine rich Repeat and Pyrin domain 1 (NLRP1), NLRP3, nucleotide-binding oligomerization domain-containing protein 1 (NOD1), and NOD2 transcripts were increased at 2 weeks and gradually reduced thereafter. Notably, NLRP3 showed the highest expression in the eyes with EAU. Similarly, the transcript level of pro-inflammatory cytokine, interleukin-1beta, increased and reached a peak at 2 weeks post-immunization. The retinal structure was severely damaged by inflammation at 3 weeks post-immunization.
CONCLUSIONS
Among NLRs, NLRP3 may induce inflammation in eyes after EAU immunization.
MeSH Terms
Figure
Reference
-
References
1. Chang JH, Wakefield D. Uveitis: a global perspective. Ocul Immunol Inflamm. 2002; 10:263–79.
Article2. Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010; 120:3073–83.
Article3. Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005; 26:447–54.
Article4. Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006; 126:659–62.
Article5. Caspi RR. Experimental autoimmune uveoretinitis in the rat and mouse. Curr Protoc Immunol. 2003; Chapter 15:Unit 15.6.
Article6. Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004; 111:491–500. discussion 500.
Article7. LeHoang P. The gold standard of noninfectious uveitis: corti- costeroids. Dev Ophthalmol. 2012; 51:7–28.8. Willermain F, Rosenbaum JT, Bodaghi B. . Interplay between innate and adaptive immunity in the development of non-infectious uveitis. Prog Retin Eye Res. 2012; 31:182–94.
Article9. Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009; 27:229–65.
Article10. Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006; 6:9–20.
Article11. Vandanmagsar B, Youm YH, Ravussin A. . The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011; 17:179–88.
Article12. Rosenzweig HL, Planck SR, Rosenbaum JT. NLRs in immune privileged sites. Curr Opin Pharmacol. 2011; 11:423–8.
Article13. Franchi L, Warner N, Viani K, Nuñez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009; 227:106–28.
Article14. Chen M, Wang H, Chen W, Meng G. Regulation of adaptive immunity by the NLRP3 inflammasome. Int Immunopharmacol. 2011; 11:549–54.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Cyclosporin A on the Recurrence in Experimental Autoimmune Anterior Uveitis
- Defects in the differentiation and function of bone marrow-derived dendritic cells in non-obese diabetic mice
- Blood-retina barrier dysfunction in experimental autoimmune uveitis: the pathogenesis and therapeutic targets
- Expression of Cell Surface Receptors on Human Glioblastoma Xenograft Model in NOD/SCID Mouse
- Experimental Autoimmune Uveitis induced by Bovine Iris and Ciliary body in Lewis Rat