Anat Cell Biol.
2022 Mar;55(1):20-27. 10.5115/acb.21.227.
Blood-retina barrier dysfunction in experimental autoimmune uveitis: the pathogenesis and therapeutic targets
- Affiliations
-
- 1Department of Anatomy, Kosin University College of Medicine, Busan,
- 2Department of Veterinary Anatomy, College of Veterinary Medicine and Veterinary Medical Research Institute, Jeju National University, Jeju,
- 3Department of Animal Science, College of Life Science, Sangji University, Wonju,
- 4Functional Biomaterials Research Center, Korea Research Institute of Bioscience and Biotechnology, Jeongeup,
- 5Department of Veterinary Anatomy, College of Veterinary Medicine and BK21 Plus Project Team, Chonnam National University, Gwangju, Korea
- KMID: 2527665
- DOI: http://doi.org/10.5115/acb.21.227
Abstract
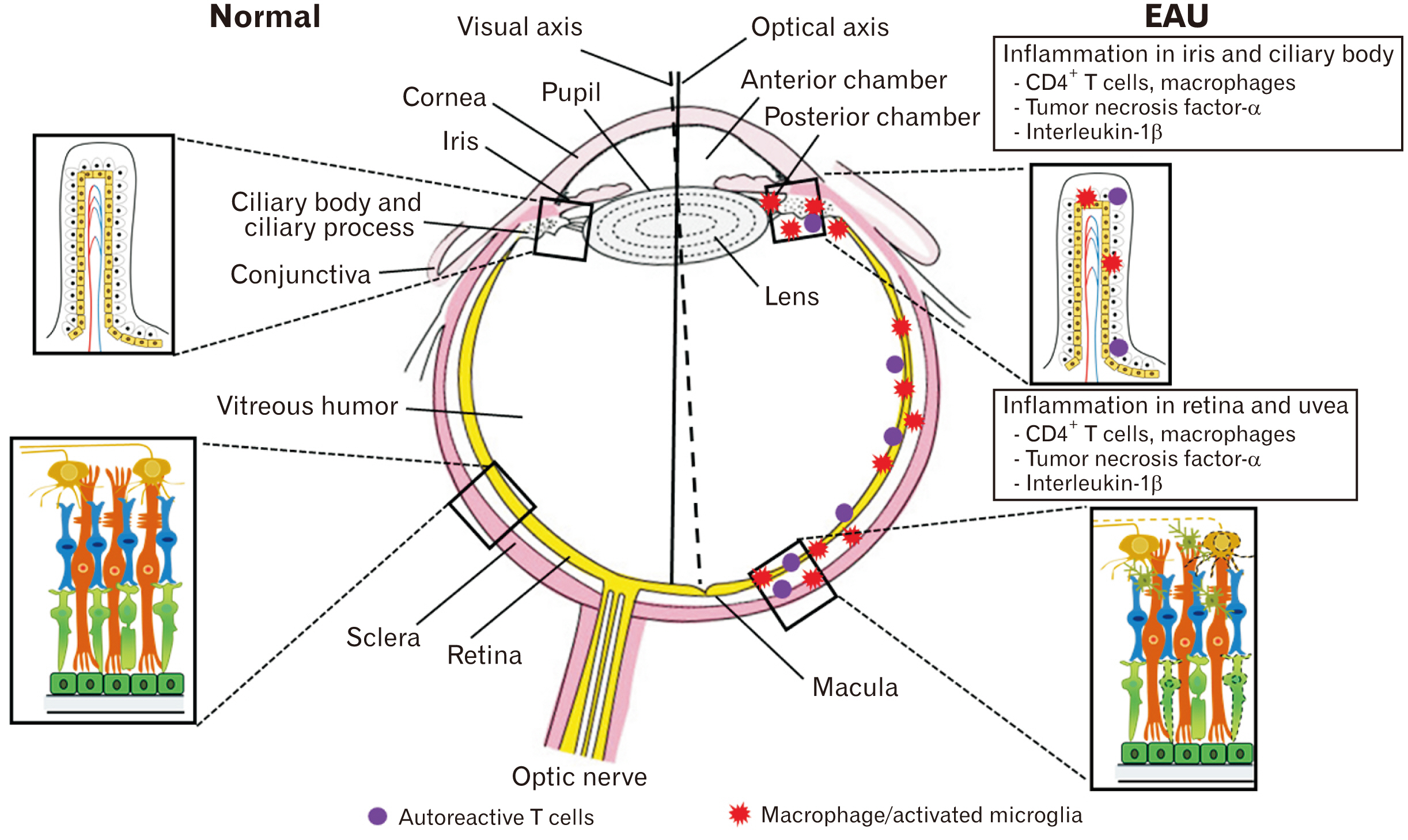
- Experimental autoimmune uveitis (EAU), an animal model of human uveitis, is characterized by infiltration of autoimmune T cells in the uvea as well as in the retina of susceptible animals. EAU is induced by the immunization of uveitogenic antigens, including either retinal soluble-antigen or interphotoreceptor retinoid-binding proteins, in Lewis rats. The pathogenesis of EAU in rats involves the proliferation of autoimmune T cells in peripheral lymphoid tissues and breakdown of the blood-retinal barrier, primarily in the uvea and retina, finally inducing visual dysfunction. In this review, we describe recent EAU studies to facilitate the design of a therapeutic strategy through the interruption of uveitogenic factors during the course of EAU, which will be helpful for controlling human uveitis.
Keyword
Figure
Reference
-
References
1. Jabs DA. 2018; Immunosuppression for the uveitides. Ophthalmology. 125:193–202. DOI: 10.1016/j.ophtha.2017.08.007. PMID: 28942074. PMCID: PMC5794515.
Article2. Chmielewski NT, Brooks DE, Smith PJ, Hendrix DV, Whittaker C, Gelatt KN. 1997; Visual outcome and ocular survival following iris prolapse in the horse: a review of 32 cases. Equine Vet J. 29:31–9. DOI: 10.1111/j.2042-3306.1997.tb01633.x. PMID: 9031861.
Article3. de Smet MD, Taylor SR, Bodaghi B, Miserocchi E, Murray PI, Pleyer U, Zierhut M, Barisani-Asenbauer T, LeHoang P, Lightman S. 2011; Understanding uveitis: the impact of research on visual outcomes. Prog Retin Eye Res. 30:452–70. DOI: 10.1016/j.preteyeres.2011.06.005. PMID: 21807112.
Article4. Mattapallil MJ, Silver PB, Mattapallil JJ, Horai R, Karabekian Z, McDowell JH, Chan CC, James EA, Kwok WW, Sen HN, Nussenblatt RB, David CS, Caspi RR. 2011; Uveitis-associated epitopes of retinal antigens are pathogenic in the humanized mouse model of uveitis and identify autoaggressive T cells. J Immunol. 187:1977–85. DOI: 10.4049/jimmunol.1101247. PMID: 21765017. PMCID: PMC3150271.
Article5. Wildner G, Diedrichs-Mohring M, Thurau SR. 2008; Rat models of autoimmune uveitis. Ophthalmic Res. 40:141–4. DOI: 10.1159/000119865. PMID: 18421228.
Article6. Caspi RR. 2014; Understanding autoimmunity in the eye: from animal models to novel therapies. Discov Med. 17:155–62. PMID: 24641958. PMCID: PMC4573559.7. Klaska IP, Forrester JV. 2015; Mouse models of autoimmune uveitis. Curr Pharm Des. 21:2453–67. DOI: 10.2174/1381612821666150316122928. PMID: 25777760.
Article8. Diedrichs-Möhring M, Kaufmann U, Wildner G. 2018; The immunopathogenesis of chronic and relapsing autoimmune uveitis - lessons from experimental rat models. Prog Retin Eye Res. 65:107–26. DOI: 10.1016/j.preteyeres.2018.02.003. PMID: 29496590.
Article9. Kyger M, Worley A, Huan J, McDowell H, Smith WC, Burrows GG, Mattapallil MJ, Caspi RR, Adamus G. 2013; Effective arrestin-specific immunotherapy of experimental autoimmune uveitis with RTL: a prospect for treatment of human uveitis. Transl Vis Sci Technol. 2:1. DOI: 10.1167/tvst.2.2.1. PMID: 24049712. PMCID: PMC3763891.
Article10. Agarwal RK, Silver PB, Caspi RR. 2012; Rodent models of experimental autoimmune uveitis. Methods Mol Biol. 900:443–69. DOI: 10.1007/978-1-60761-720-4_22. PMID: 22933083. PMCID: PMC3810964.
Article11. Kang J, Ahn M, Moon C, Min DS, Matsumoto Y, Shin T. 2005; Phospholipase D1 is up-regulated in the retina of Lewis rats with experimental autoimmune uveoretinitis. Immunol Invest. 34:27–36. DOI: 10.1081/imm-200047381. PMID: 15773570.
Article12. Caspi RR. 2003; Experimental autoimmune uveoretinitis in the rat and mouse. Curr Protoc Immunol. Chapter 15:Unit 15.6. DOI: 10.1002/0471142735.im1506s53. PMID: 18432901.
Article13. Egwuagu CE, Mahdi RM, Nussenblatt RB, Gery I, Caspi RR. 1993; Evidence for selective accumulation of V beta 8+ T lymphocytes in experimental autoimmune uveoretinitis induced with two different retinal antigens. J Immunol. 151:1627–36. PMID: 8393049.14. Diedrichs-Möhring M, Hoffmann C, Wildner G. 2008; Antigen-dependent monophasic or recurrent autoimmune uveitis in rats. Int Immunol. 20:365–74. DOI: 10.1093/intimm/dxm148. PMID: 18203685.
Article15. Yin X, Liu B, Wei H, Wu S, Guo L, Xu F, Liu T, Bi H, Guo D. 2019; Activation of the Notch signaling pathway disturbs the CD4+/CD8+, Th17/Treg balance in rats with experimental autoimmune uveitis. Inflamm Res. 68:761–74. DOI: 10.1007/s00011-019-01260-w. PMID: 31209505.
Article16. Jia X, Hu M, Wang C, Wang C, Zhang F, Han Q, Zhao R, Huang Q, Xu H, Yuan H, Ren H. 2011; Coordinated gene expression of Th17- and Treg-associated molecules correlated with resolution of the monophasic experimental autoimmune uveitis. Mol Vis. 17:1493–507. PMID: 21686325. PMCID: PMC3115744.17. Kim G, Kohyama K, Tanuma N, Arimito H, Matsumoto Y. 1998; Persistent expression of experimental autoimmune encephalomyelitis (EAE)-specific Vbeta8.2 TCR spectratype in the central nervous system of rats with chronic relapsing EAE. J Immunol. 161:6993–8. PMID: 9862735.18. Buenafe AC, Offner H, Machnicki M, Elerding H, Adlard K, Jacobs R, Vandenbark AA, Adamus G. 1998; EAE TCR motifs and antigen recognition in myelin basic protein-induced anterior uveitis in Lewis rats. J Immunol. 161:2052–9. PMID: 9712079.19. Chan CC, Caspi R, Mochizuki M, Diamantstein T, Gery I, Nussenblatt RB. 1987; Cyclosporine and dexamethasone inhibit T-lymphocyte MHC class II antigens and IL-2 receptor expression in experimental autoimmune uveitis. Immunol Invest. 16:319–31. DOI: 10.3109/08820138709087087. PMID: 3123380.
Article20. Lobanoff MC, Kozhich AT, Mullet DI, Gerber N, Gery I, Chan CC, Whitcup SM. 1997; Effect of gallium nitrate on experimental autoimmune uveitis. Exp Eye Res. 65:797–801. DOI: 10.1006/exer.1997.0395. PMID: 9441703.
Article21. Toriyama S. 2000; Effects of leukotriene B(4) receptor antagonist on experimental autoimmune uveoretinitis in rats. Jpn J Ophthalmol. 44:695. DOI: 10.1016/s0021-5155(00)00262-8. PMID: 11094206.
Article22. Liang W, Karabekian Z, Mattapallil M, Xu Q, Viley AM, Caspi R, Scott DW. 2006; B-cell delivered gene transfer of human S-Ag-Ig fusion protein protects from experimental autoimmune uveitis. Clin Immunol. 118:35–41. Erratum in: Clin Immunol 2006;120:357. DOI: 10.1016/j.clim.2005.08.007. PMID: 16168712.
Article23. Sakai T, Kohno H, Ishihara T, Higaki M, Saito S, Matsushima M, Mizushima Y, Kitahara K. 2006; Treatment of experimental autoimmune uveoretinitis with poly(lactic acid) nanoparticles encapsulating betamethasone phosphate. Exp Eye Res. 82:657–63. DOI: 10.1016/j.exer.2005.09.003. PMID: 16360654.
Article24. Osada M, Sakai T, Kuroyanagi K, Kohno H, Tsuneoka H. 2014; Treatment of experimental autoimmune uveoretinitis with peroxisome proliferator-activated receptor α agonist fenofibrate. Mol Vis. 20:1518–26. PMID: 25489225. PMCID: PMC4225135.25. Kim J, Ahn M, Choi Y, Chun J, Jung K, Tanaka A, Matsuda H, Shin T. 2022; Osteopontin is a biomarker for early autoimmune uveoretinitis. Neural Regen Res. 17:1604–8. DOI: 10.4103/1673-5374.330614. PMID: 34916447. PMCID: PMC8771122. PMID: e6f5553cb10f43f9a532d74599473734.
Article26. Dick AD, Cheng YF, Liversidge J, Forrester JV. 1994; Immunomodulation of experimental autoimmune uveoretinitis: a model of tolerance induction with retinal antigens. Eye (Lond). 8(Pt 1):52–9. DOI: 10.1038/eye.1994.10. PMID: 8013720.
Article27. Greenwood J, Howes R, Lightman S. 1994; The blood-retinal barrier in experimental autoimmune uveoretinitis. Leukocyte interactions and functional damage. Lab Invest. 70:39–52. PMID: 8302017.28. Rao NA, Kimoto T, Zamir E, Giri R, Wang R, Ito S, Pararajasegaram G, Read RW, Wu GS. 2003; Pathogenic role of retinal microglia in experimental uveoretinitis. Invest Ophthalmol Vis Sci. 44:22–31. DOI: 10.1167/iovs.02-0199. PMID: 12506051.
Article29. Chiba C. 2014; The retinal pigment epithelium: an important player of retinal disorders and regeneration. Exp Eye Res. 123:107–14. DOI: 10.1016/j.exer.2013.07.009. PMID: 23880527.
Article30. Luna JD, Chan CC, Derevjanik NL, Mahlow J, Chiu C, Peng B, Tobe T, Campochiaro PA, Vinores SA. 1997; Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: comparison with vascular endothelial growth factor, tumor necrosis factor alpha, and interleukin-1beta-mediated breakdown. J Neurosci Res. 49:268–80. DOI: 10.1002/(sici)1097-4547(19970801)49:3<268::aid-jnr2>3.0.co;2-a. PMID: 9260738.31. Prendergast RA, Iliff CE, Coskuncan NM, Caspi RR, Sartani G, Tarrant TK, Lutty GA, McLeod DS. 1998; T cell traffic and the inflammatory response in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 39:754–62. PMID: 9538882.32. Tyler NK, Burns MS. 1991; Comparison of lectin reactivity in the vessel beds of the rat eye. Curr Eye Res. 10:801–10. DOI: 10.3109/02713689109013875. PMID: 1790711.
Article33. Dick AD, Forrester JV, Liversidge J, Cope AP. 2004; The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU). Prog Retin Eye Res. 23:617–37. DOI: 10.1016/j.preteyeres.2004.06.005. PMID: 15388077.34. Pavan B, Dalpiaz A. 2018; Retinal pigment epithelial cells as a therapeutic tool and target against retinopathies. Drug Discov Today. 23:1672–9. DOI: 10.1016/j.drudis.2018.06.009. PMID: 29908265.
Article35. Lee H, Hwang-Bo H, Ji SY, Kim MY, Kim SY, Park C, Hong SH, Kim GY, Song KS, Hyun JW, Choi YH. 2020; Diesel particulate matter2.5 promotes epithelial-mesenchymal transition of human retinal pigment epithelial cells via generation of reactive oxygen species. Environ Pollut. 262:114301. DOI: 10.1016/j.envpol.2020.114301. PMID: 32155554.
Article36. Giebel SJ, Menicucci G, McGuire PG, Das A. 2005; Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest. 85:597–607. DOI: 10.1038/labinvest.3700251. PMID: 15711567.
Article37. Zhang R, Yang PZ, Wu CY, Jin HL, Li B, Huang XK, Zhou HY, Gao Y, Zhu LX, Kijlstra A. 2006; Role of T-cell receptor V beta 8.3 peptide vaccine in the prevention of experimental autoimmune uveoretinitis. Chin Med J (Engl). 119:740–8. DOI: 10.1097/00029330-200605010-00006. PMID: 16701014.
Article38. Wu W, Zhang Z, Xiong T, Zhao W, Jiang R, Chen H, Li X. 2017; Calcium ion coordinated dexamethasone supramolecular hydrogel as therapeutic alternative for control of non-infectious uveitis. Acta Biomater. 61:157–68. DOI: 10.1016/j.actbio.2017.05.024. PMID: 28501709.
Article39. Robertson M, Liversidge J, Forrester JV, Dick AD. 2003; Neutralizing tumor necrosis factor-alpha activity suppresses activation of infiltrating macrophages in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 44:3034–41. DOI: 10.1167/iovs.02-1156. PMID: 12824249.
Article40. Kwak HJ, Yang YS, Pae HO, Kim YM, Chung HT. 2001; Exogenous nitric oxide inhibits experimental autoimmune uveoretinitis development in Lewis rats by modulation of the Th1-dependent immune response. Mol Cells. 12:178–84. PMID: 11710518.41. Sakai T, Ishihara T, Higaki M, Akiyama G, Tsuneoka H. 2011; Therapeutic effect of stealth-type polymeric nanoparticles with encapsulated betamethasone phosphate on experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 52:1516–21. DOI: 10.1167/iovs.10-5676. PMID: 21178146.
Article42. Johnsen-Soriano S, Sancho-Tello M, Arnal E, Díaz-Llopis M, Navea A, Miranda M, Bosch-Morell F, Romero FJ. 2010; Comparison of the acute effects of anti-TNF-alpha drugs on a uveitis experimental model. Ocul Immunol Inflamm. 18:208–15. DOI: 10.3109/09273940903521964. PMID: 20482400.
Article43. Crupi R, Impellizzeri D, Gugliandolo E, Cordaro M, Siracusa R, Britti D, Cuzzocrea S, Di Paola R. 2019; Effect of Tempol, a membrane-permeable free radical scavenger, on in vitro model of eye inflammation on rabbit corneal cells. J Ocul Pharmacol Ther. 35:571–7. DOI: 10.1089/jop.2019.0016. PMID: 31825758.44. Rajendram R, Saraswathy S, Rao NA. 2007; Photoreceptor mitochondrial oxidative stress in early experimental autoimmune uveoretinitis. Br J Ophthalmol. 91:531–7. DOI: 10.1136/bjo.2006.101576. PMID: 17035279. PMCID: PMC1994769.
Article45. Harzallah O, Kerkeni A, Baati T, Mahjoub S. 2008; Oxidative stress: correlation with Behçet’s disease duration, activity and severity. Eur J Intern Med. 19:541–7.
Article46. Augustin AJ, Loeffler KU, Sekundo W, Grus FH, Lutz J. 1999; Effects of systemically applied allopurinol and prednisolone on experimental autoimmune uveitis. Graefes Arch Clin Exp Ophthalmol. 237:508–12. DOI: 10.1007/s004170050270. PMID: 10379613.
Article47. Sasaki H, Lin LR, Yokoyama T, Sevilla MD, Reddy VN, Giblin FJ. 1998; TEMPOL protects against lens DNA strand breaks and cataract in the x-rayed rabbit. Invest Ophthalmol Vis Sci. 39:544–52. PMID: 9501865.48. Zamir E, Zhang R, Samuni A, Kogan M, Pe’er J. 1999; Nitroxide stable radical suppresses autoimmune uveitis in rats. Free Radic Biol Med. 27:7–15. DOI: 10.1016/s0891-5849(99)00026-x. PMID: 10443914.
Article49. Yadav UC, Shoeb M, ivastava SK Sr, Ramana KV. 2011; Amelioration of experimental autoimmune uveoretinitis by aldose reductase inhibition in Lewis rats. Invest Ophthalmol Vis Sci. 52:8033–41. DOI: 10.1167/iovs.11-7485. PMID: 21900376. PMCID: PMC3208000.
Article50. Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. 2005; Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 436:266–71. DOI: 10.1038/nature03889. PMID: 16015332.
Article51. Uchio E, Kijima M, Tanaka S, Ohno S. 1994; Suppression of experimental uveitis with monoclonal antibodies to ICAM-1 and LFA-1. Invest Ophthalmol Vis Sci. 35:2626–31. PMID: 7909311.52. Kitaichi N, Matsuda A, Kotake S, Namba K, Tagawa Y, Sasamoto Y, Ogasawara K, Iwabuchi K, Onoé K, Matsuda H, Nishihira J. 2000; Inhibition of experimental autoimmune uveoretinitis with anti-macrophage migration inhibitory factor antibodies. Curr Eye Res. 20:109–14. DOI: 10.1076/0271-3683(200002)2021-dft109. PMID: 10617911.
Article53. Wallace GR, Whiston RA, Stanford MR, Wells GM, Gearing AJ, Clements JM. 1999; The matrix metalloproteinase inhibitor BB-1101 prevents experimental autoimmune uveoretinitis (EAU). Clin Exp Immunol. 118:364–70. DOI: 10.1046/j.1365-2249.1999.01066.x. PMID: 10594553. PMCID: PMC1905444.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tne effets of anti-inflammatory ageets on bloed-aqueous-barrier in experimental uveitis
- Effects of Cyclosporin A on the Recurrence in Experimental Autoimmune Anterior Uveitis
- Experimental Autoimmune Uveitis induced by Bovine Iris and Ciliary body in Lewis Rat
- Blood-brain barrier dysfunction in ischemic stroke and diabetes: the underlying link, mechanisms and future possible therapeutic targets
- Direct Detection of Reactive Nitrogen Species in Experimental Autoimmune Uveitis