J Korean Surg Soc.
2013 Apr;84(4):216-224. 10.4174/jkss.2013.84.4.216.
Injection of porous polycaprolactone beads containing autologous myoblasts in a dog model of fecal incontinence
- Affiliations
-
- 1Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 2Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 3Department of Rehabilitation, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 4Department of Advanced Materials, Hannam University, Daejeon, Korea. jhlee@hnu.kr
- KMID: 2212466
- DOI: http://doi.org/10.4174/jkss.2013.84.4.216
Abstract
- PURPOSE
Few studies have examined whether bioengineering can improve fecal incontinence. This study designed to determine whether injection of porous polycaprolactone beads containing autologous myoblasts improves sphincter function in a dog model of fecal incontinence.
METHODS
The anal sphincter of dogs was injured and the dogs were observed without and with (n = 5) the injection of porous polycaprolactone beads containing autologous myoblasts into the site of injury. Autologous myoblasts purified from the gastrocnemius muscles were transferred to the beads. Compound muscle action potentials (CMAP) of the pudendal nerve, anal sphincter pressure, and histopathology were determined 3 months after treatment.
RESULTS
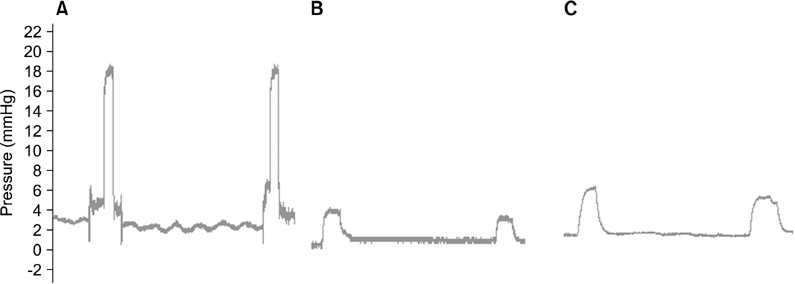
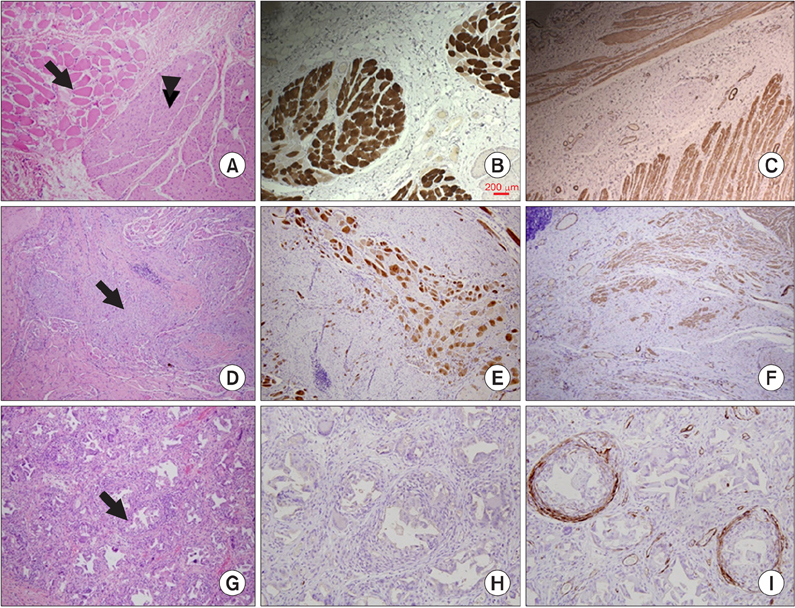
The amplitudes of the CMAP in the injured sphincter were significantly lower than those measured before injury (1.22 mV vs. 3.00 mV, P = 0.04). The amplitudes were not different between dogs with and without the injection of autologous myoblast beads (P = 0.49). Resting and squeezing pressures were higher in dogs treated with autologous myoblast beads (2.00 mmHg vs. 1.80 mmHg; 6.13 mmHg vs. 4.02 mmHg), although these differences were not significant in analyses of covariance adjusted for baseline values. The injection site was stained for smooth muscle actin, but showed evidence of foreign body inflammatory reactions.
CONCLUSION
This was the first study to examine whether bioengineering could improve fecal incontinence. Although the results did not show definite evidence that injection of autologous myoblast beads improves sphincter function, we found that the dog model was suitable and reliable for studying the effects of a potential treatment modality for fecal incontinence.
Keyword
MeSH Terms
Figure
Reference
-
1. Nelson R, Norton N, Cautley E, Furner S. Community-based prevalence of anal incontinence. JAMA. 1995. 274:559–561.2. Macmillan AK, Merrie AE, Marshall RJ, Parry BR. The prevalence of fecal incontinence in community-dwelling adults: a systematic review of the literature. Dis Colon Rectum. 2004. 47:1341–1349.3. Roberts RO, Jacobsen SJ, Reilly WT, Pemberton JH, Lieber MM, Talley NJ. Prevalence of combined fecal and urinary incontinence: a community-based study. J Am Geriatr Soc. 1999. 47:837–841.4. Ito M, Saito N, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y. Analysis of clinical factors associated with anal function after intersphincteric resection for very low rectal cancer. Dis Colon Rectum. 2009. 52:64–70.5. Wald A. Clinical practice: fecal incontinence in adults. N Engl J Med. 2007. 356:1648–1655.6. Shafik A. Polytetrafluoroethylene injection for the treatment of partial fecal incontinence. Int Surg. 1993. 78:159–161.7. Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001. 166:1350–1353.8. Malizia AA Jr, Reiman HM, Myers RP, Sande JR, Barham SS, Benson RC Jr, et al. Migration and granulomatous reaction after periurethral injection of polytef (Teflon). JAMA. 1984. 251:3277–3281.9. Williams NS. Pelvic floor disorders and reconstruction: what next? Dis Colon Rectum. 2008. 51:1309–1311.10. Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005. 48:1416–1423.11. Lorenzi B, Pessina F, Lorenzoni P, Urbani S, Vernillo R, Sgaragli G, et al. Treatment of experimental injury of anal sphincters with primary surgical repair and injection of bone marrow-derived mesenchymal stem cells. Dis Colon Rectum. 2008. 51:411–420.12. Kang SB, Lee HN, Lee JY, Park JS, Lee HS, Lee JY. Sphincter contractility after muscle-derived stem cells autograft into the cryoinjured anal sphincters of rats. Dis Colon Rectum. 2008. 51:1367–1373.13. Salcedo L, Damaser M, Butler R, Jiang HH, Hull T, Zutshi M. Long-term effects on pressure and electromyography in a rat model of anal sphincter injury. Dis Colon Rectum. 2010. 53:1209–1217.14. Zutshi M, Salcedo LB, Zaszczurynski PJ, Hull TL, Butler RS, Damaser MS. Effects of sphincterotomy and pudendal nerve transection on the anal sphincter in a rat model. Dis Colon Rectum. 2009. 52:1321–1329.15. Kajbafzadeh AM, Elmi A, Talab SS, Esfahani SA, Tourchi A. Functional external anal sphincter reconstruction for treatment of anal incontinence using muscle progenitor cell auto grafting. Dis Colon Rectum. 2010. 53:1415–1421.16. Frudinger A, Kolle D, Schwaiger W, Pfeifer J, Paede J, Halligan S. Muscle-derived cell injection to treat anal incontinence due to obstetric trauma: pilot study with 1 year follow-up. Gut. 2010. 59:55–61.17. Kang SB, Lee TG. Muscle regeneration: research for the treatment of fecal incontinence. J Korean Soc Coloproctol. 2010. 26:1–7.18. Lim SM, Lee HJ, Oh SH, Kim JM, Lee JH. Novel fabrication of PCL porous beads for use as an injectable cell carrier system. J Biomed Mater Res B Appl Biomater. 2009. 90:521–530.19. Oh SH, Kim IG, Lee JY, Lee JY, Lee JH. Bioactive porous beads as an injectable urethral bulking agent: their in vitro evaluation on smooth muscle cell differentiation. Tissue Eng Part A. 2011. 17:655–664.20. Kim IG, Oh SH, Lee JY, Lee JY, Lee JH. Bioactive porous beads as an injectable urethral bulking agent: in vivo animal study for the treatment of urinary incontinence. Tissue Eng Part A. 2011. 17:1527–1535.21. Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994. 125:1275–1287.22. Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008. 29:2899–2906.23. Saihara R, Komuro H, Urita Y, Hagiwara K, Kaneko M. Myoblast transplantation to defecation muscles in a rat model: a possible treatment strategy for fecal incontinence after the repair of imperforate anus. Pediatr Surg Int. 2009. 25:981–986.24. Nijhuis PH, van den Bogaard TE, Daemen MJ, Baeten CG. Perianal injection of polydimethylsiloxane (Bioplastique implants) paste in the treatment of soiling: pilot study in rats to determine migratory tendency and locoregional reaction. Dis Colon Rectum. 1998. 41:624–629.25. Maeda Y, Laurberg S, Norton C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst Rev. 2010. (5):CD007959.26. Alameddine HS, Louboutin JP, Dehaupas M, Sebille A, Fardeau M. Functional recovery induced by satellite cell grafts in irreversibly injured muscles. Cell Transplant. 1994. 3:3–14.27. Guettier-Sigrist S, Coupin G, Warter JM, Poindron P. Cell types required to efficiently innervate human muscle cells in vitro. Exp Cell Res. 2000. 259:204–212.28. Culver PJ, Rattan S. Genesis of anal canal pressures in the opossum. Am J Physiol. 1986. 251(6 Pt 1):G765–G771.29. Kimura J. Electrodiagnosis in disease of nerve and muscle. 2001. Oxford: Oxford Univ ersity Press.30. Yazici I, Ayhan S, Elmas C, Temucin C, Atabay K. Motor neurotization by segmental epineurectomy and implantation: lateral muscular neurotization. J Reconstr Microsurg. 2008. 24:435–442.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fecal Incontinence as a Symptom of Myasthenia Gravis
- The Initial Experience of Endoscopic Periurethral Autologous Fat Injection in Stress Urinary Incontinence
- Role of Permacol Injection in the Treatment of Patients With Fecal Incontinence
- Muscle Regeneration: Research for the Treatment of Fecal Incontinence
- Culture of Rabbit Chondrocytes Using Chitosan Bead