J Periodontal Implant Sci.
2012 Oct;42(5):158-165. 10.5051/jpis.2012.42.5.158.
Changes in periodontium after extraction of a periodontally-involved tooth in rats
- Affiliations
-
- 1Department of Periodontology, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- 2Department of Conservative Dentistry, Yonsei University College of Dentistry, Seoul, Korea.
- KMID: 2212075
- DOI: http://doi.org/10.5051/jpis.2012.42.5.158
Abstract
- PURPOSE
Recent interest has focused on intentional replantation to restore an original tooth. Some studies have shown successful results with intentional replantation for periodontally involved teeth. For long-term success of replantation, a healthy periodontal status of the recipient site is required so that delayed replantation is more suitable for periodontally involved teeth. To reveal the ideal timing for delayed replantation of periodontally involved teeth, the healing process of extraction sockets after extraction of periodontitis-induced teeth in rats was evaluated.
METHODS
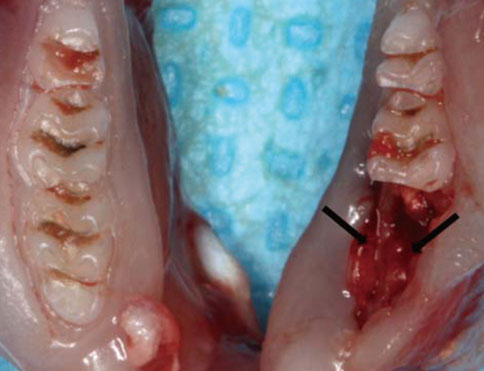
Twenty-eight rats were randomly divided into two groups: a control group (n=8) and test group (n=20). In the test group, periodontitis was induced by a ligature around the cervix of the mandibular first molar of all of the rats. Two weeks later, the mandibular first molars were extracted in all of the animals. The animals were sacrificed on days 0, 3, 7, and 10 after extraction and histological and immunohistochemical analysis was performed.
RESULTS
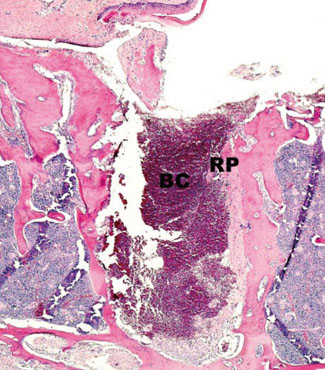
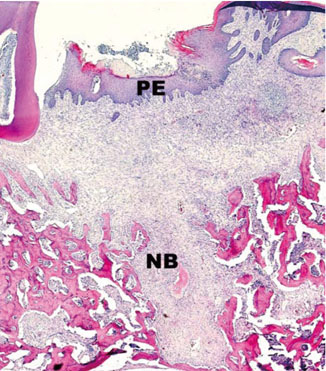
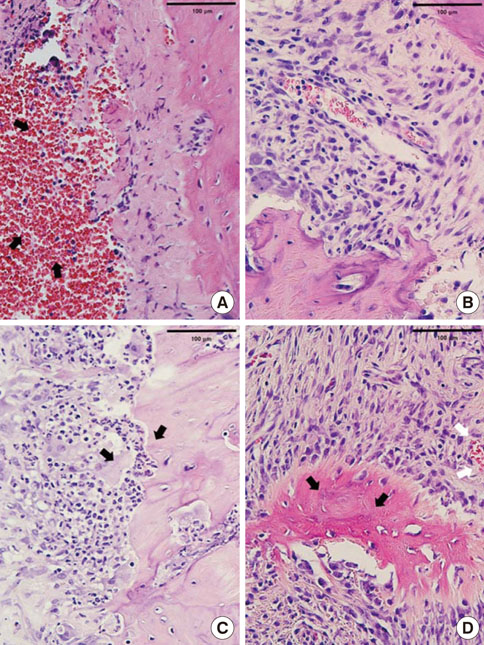
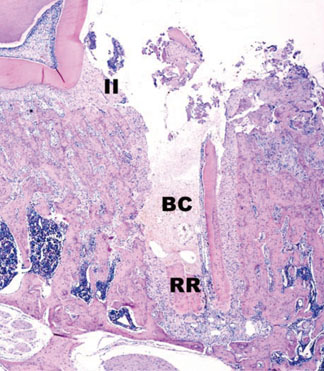
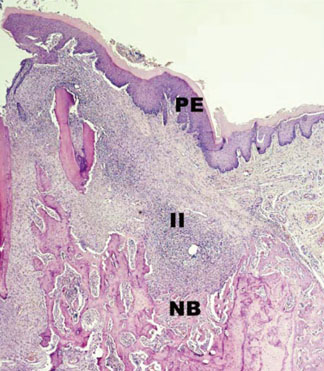
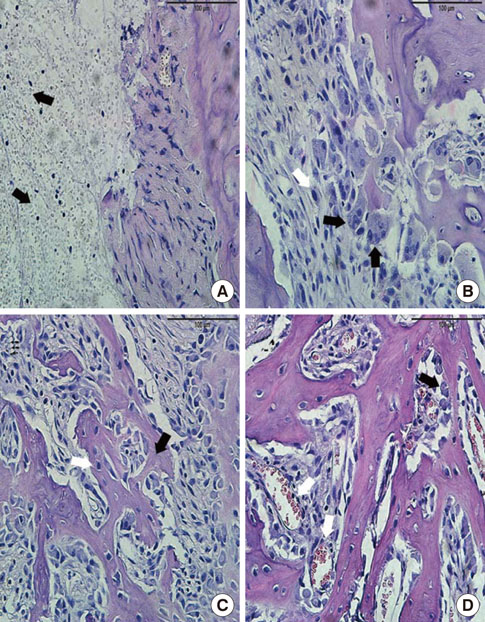
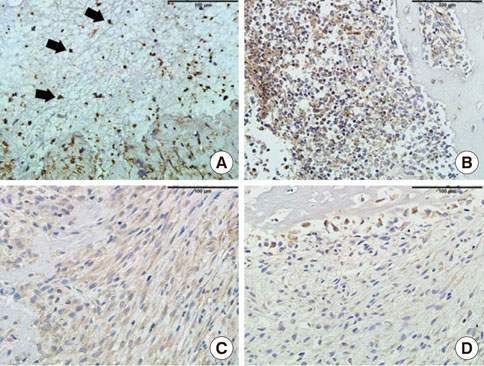
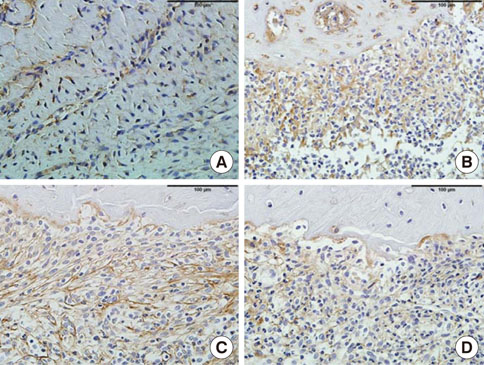
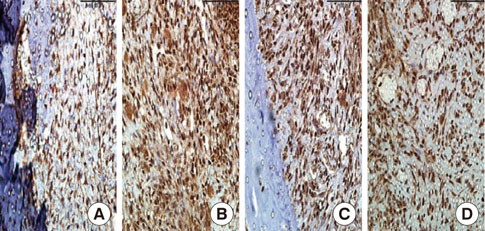
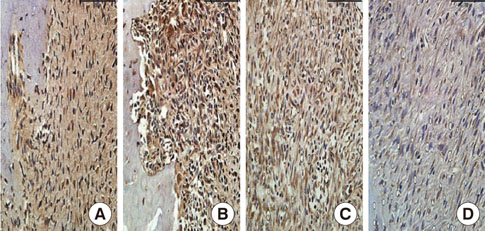
In histological analysis of the test group, inflammatory cell infiltrate was found abundantly in the remaining periodontium 3 days after tooth extraction and decreased gradually at later time points. In immunohistochemical analysis of the test group, both interleukin-6 (IL-6) and, tumor necrosis factor-alpha (TNF-alpha) were numerous in the furcation area at each postextraction day. IL-6 was stained more heavily between 3 and 7 days after extraction; at day 10 after extraction, little staining was observed. TNF-alpha staining was more intense at 3 days after extraction and gradually weakened at later points in time.
CONCLUSIONS
Within the limits of this study, it takes at least 10 days to resolve periodontal inflammation in rat extraction sockets.
MeSH Terms
Figure
Cited by 1 articles
-
Delayed intentional replantation of periodontally hopeless teeth: a retrospective study
Eun-Ung Lee, Hyun-Chang Lim, Jung-Seok Lee, Ui-Won Jung, Ui-Sung Kim, Seung-Jong Lee, Seong-Ho Choi
J Periodontal Implant Sci. 2014;44(1):13-19. doi: 10.5051/jpis.2014.44.1.13.
Reference
-
1. Grossman LI. Intentional replantation of teeth: a clinical evaluation. J Am Dent Assoc. 1982. 104:633–639.
Article2. Lu DP. Intentional replantation of periodontally involved and endodontically mistreated tooth. Oral Surg Oral Med Oral Pathol. 1986. 61:508–513.
Article3. Demiralp B, Nohutcu RM, Tepe DI, Eratalay K. Intentional replantation for periodontally involved hopeless teeth. Dent Traumatol. 2003. 19:45–51.
Article4. Hammarstrom L, Blomlof L, Lindskog S. Dynamics of dentoalveolar ankylosis and associated root resorption. Endod Dent Traumatol. 1989. 5:163–175.
Article5. Andreasen JO. Analysis of pathogenesis and topography of replacement root resorption (ankylosis) after replantation of mature permanent incisors in monkeys. Swed Dent J. 1980. 4:231–240.6. Kratchman S. Intentional replantation. Dent Clin North Am. 1997. 41:603–617.7. Dryden JA, Arens DE. Intentional replantation. A viable alternative for selected cases. Dent Clin North Am. 1994. 38:325–353.8. Sharma NK, Duggal MS. Replantation in general dental practice. Br Dent J. 1994. 176:147–151.
Article9. Messkoub M. Intentional replantation: a successful alternative for hopeless teeth. Oral Surg Oral Med Oral Pathol. 1991. 71:743–747.
Article10. Lindskog S, Pierce AM, Blomlof L, Hammarstrom L. The role of the necrotic periodontal membrane in cementum resorption and ankylosis. Endod Dent Traumatol. 1985. 1:96–101.
Article11. Mahajan SK, Sidhu SS. Periodontal ligament, extra-oral period and use of fluorides in replantation of teeth. Indian J Med Res. 1982. 75:441–445.12. Madison S. Intentional replantation. Oral Surg Oral Med Oral Pathol. 1986. 62:707–709.
Article13. Nethander G, Skoglund A, Kahnberg KE. Experimental autogenous tooth transplantation in the dog: a comparison between one- and two-stage surgical techniques. Acta Odontol Scand. 2003. 61:223–229.
Article14. Duarte PM, Tezolin KR, Figueiredo LC, Feres M, Bastos MF. Microbial profile of ligature-induced periodontitis in rats. Arch Oral Biol. 2010. 55:142–147.
Article15. Chilosi M, Mombello A, Montagna L, Benedetti A, Lestani M, Semenzato G, et al. Multimarker immunohistochemical staining of calgranulins, chloroacetate esterase, and S100 for simultaneous demonstration of inflammatory cells on paraffin sections. J Histochem Cytochem. 1990. 38:1669–1675.
Article16. Pulford KA, Sipos A, Cordell JL, Stross WP, Mason DY. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990. 2:973–980.
Article17. Ackermann MR, DeBey BM, Stabel TJ, Gold JH, Register KB, Meehan JT. Distribution of anti-CD68 (EBM11) immunoreactivity in formalin-fixed, paraffin-embedded bovine tissues. Vet Pathol. 1994. 31:340–348.
Article18. Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997. 14:33–53.
Article19. Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000. 1997. 14:216–248.
Article20. Todo H. Healing mechanism of tooth extraction wounds in rats. I. Initial cellular response to tooth extraction in rats studied with 3H-thymidine. Arch Oral Biol. 1968. 13:1421–1427.21. Sato H, Takeda Y. Proliferative activity, apoptosis, and histogenesis in the early stages of rat tooth extraction wound healing. Cells Tissues Organs. 2007. 186:104–111.
Article22. Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999. 67:2804–2809.
Article23. Borch TS, Holmstrup P, Bendtzen K, Nielsen CH. In vitro cytokine responses to periodontal pathogens: generalized aggressive periodontitis is associated with increased IL-6 response to Porphyromonas gingivalis. Scand J Immunol. 2010. 71:440–446.
Article24. Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007. 356:911–920.
Article25. Lopez Carriches C, Martínez Gonzalez JM, Donado Rodriguez M. Variations of interleukin-6 after surgical removal of lower third molars. Med Oral Patol Oral Cir Bucal. 2006. 11:E520–E526.26. Miyawaki T, Maeda S, Shimada M. Elevation of plasma interleukin-6 level in patients undergoing oral and maxillofacial surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996. 81:15–20.
Article27. Cruickshank AM, Fraser WD, Burns HJ, Van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond). 1990. 79:161–165.
Article28. Graves DT, Oskoui M, Volejnikova S, Naguib G, Cai S, Desta T, et al. Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. J Dent Res. 2001. 80:1875–1879.
Article29. Gaspersic R, Stiblar-Martincic D, Osredkar J, Skaleric U. Influence of subcutaneous administration of recombinant TNF-alpha on ligature-induced periodontitis in rats. J Periodontal Res. 2003. 38:198–203.30. Garlet GP, Cardoso CR, Campanelli AP, Ferreira BR, Avila-Campos MJ, Cunha FQ, et al. The dual role of p55 tumour necrosis factor-alpha receptor in Actinobacillus actinomycetemcomitans-induced experimental periodontitis: host protection and tissue destruction. Clin Exp Immunol. 2007. 147:128–138.
Article31. Liu R, Bal HS, Desta T, Krothapalli N, Alyassi M, Luan Q, et al. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J Dent Res. 2006. 85:510–514.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A STUDY ON THE CONFIGURATION OF THE BRISTLE FOR THE PROSTHETIC AND PERIODONTALLY INVOLVED PATIENT.
- A study on the metabolic alteration of periodontal tissue on orthodontic tooth movement in rats
- Rescue of a periodontally compromised tooth by non-surgical treatment: a case report
- A histologic study on the effect of laser on the periodontium of the orthodontically moved tooth of rat
- Effect of irradiation on wound healing after tooth extraction in the rachitic rats