J Nutr Health.
2015 Aug;48(4):327-334. 10.4163/jnh.2015.48.4.327.
Effects of (6)-gingerol, ginger component on adipocyte development and differentiation in 3T3-L1
- Affiliations
-
- 1Department of Food Service Industry, Jangan University, Gyeonggi 445-756, Korea. e.young719@jangan.ac.kr
- KMID: 2209899
- DOI: http://doi.org/10.4163/jnh.2015.48.4.327
Abstract
- PURPOSE
The objective of this study was to investigate the effects of (6)-gingerol, ginger components proliferation and adipocyte differentiation from early to lately steps.
METHODS
3T3-L1 preadipocytes were cultured. Differentiation of confluent cells was induced with dexamethasone, isobutylxanthin and insulin for 2 day and cells were cultured by medium with insulin in presence of various concentrations 0, 25, 50, 100 (micromol/L) of (6)-gingerol for 4 day. Cell viability was measured using the EZ Cytox assay kit. In addition, we examined the expression of mRNA levels associated with each adipocyte differentiation step by real time reverse transcription polymerase chain reaction.
RESULTS
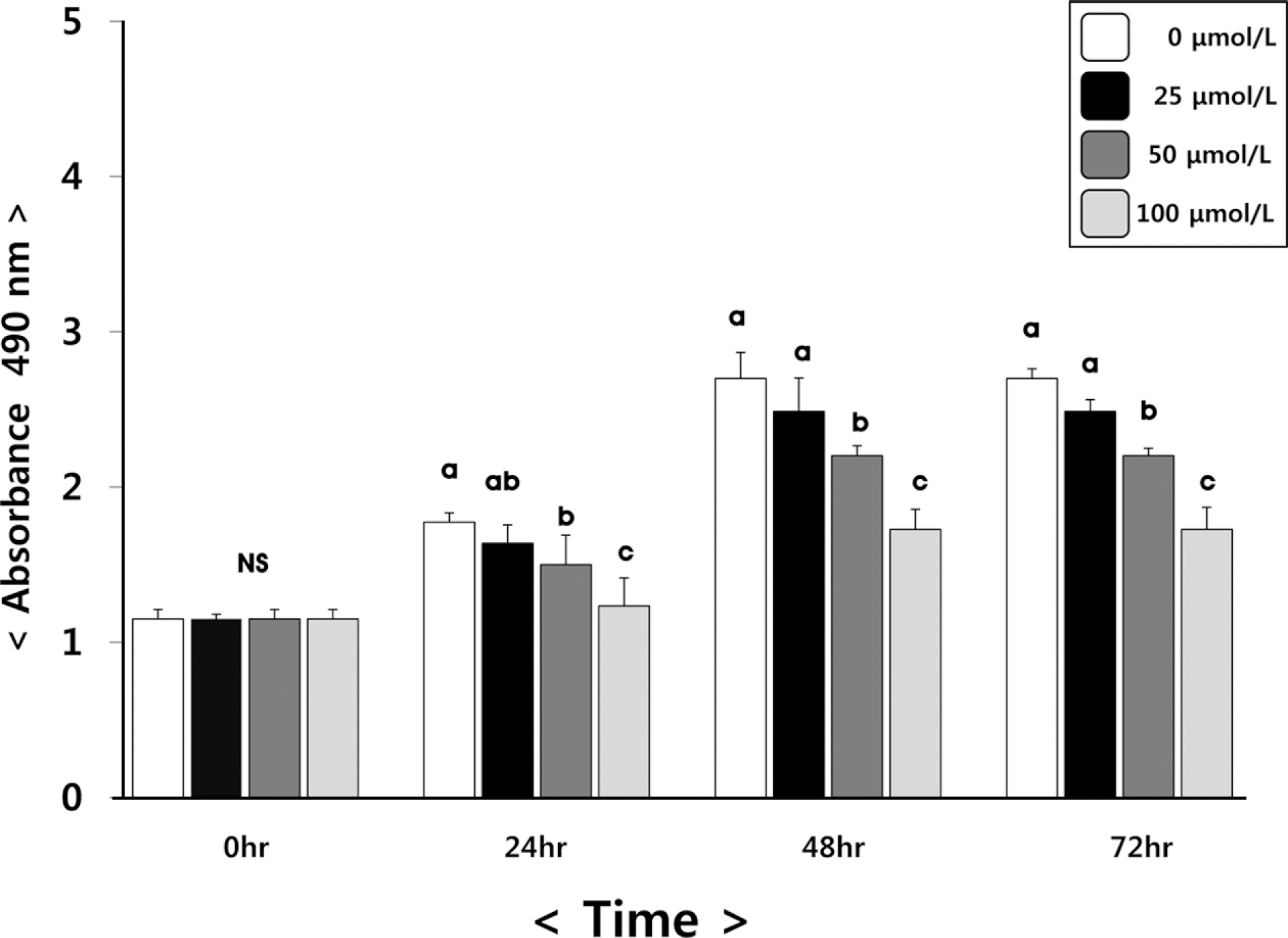
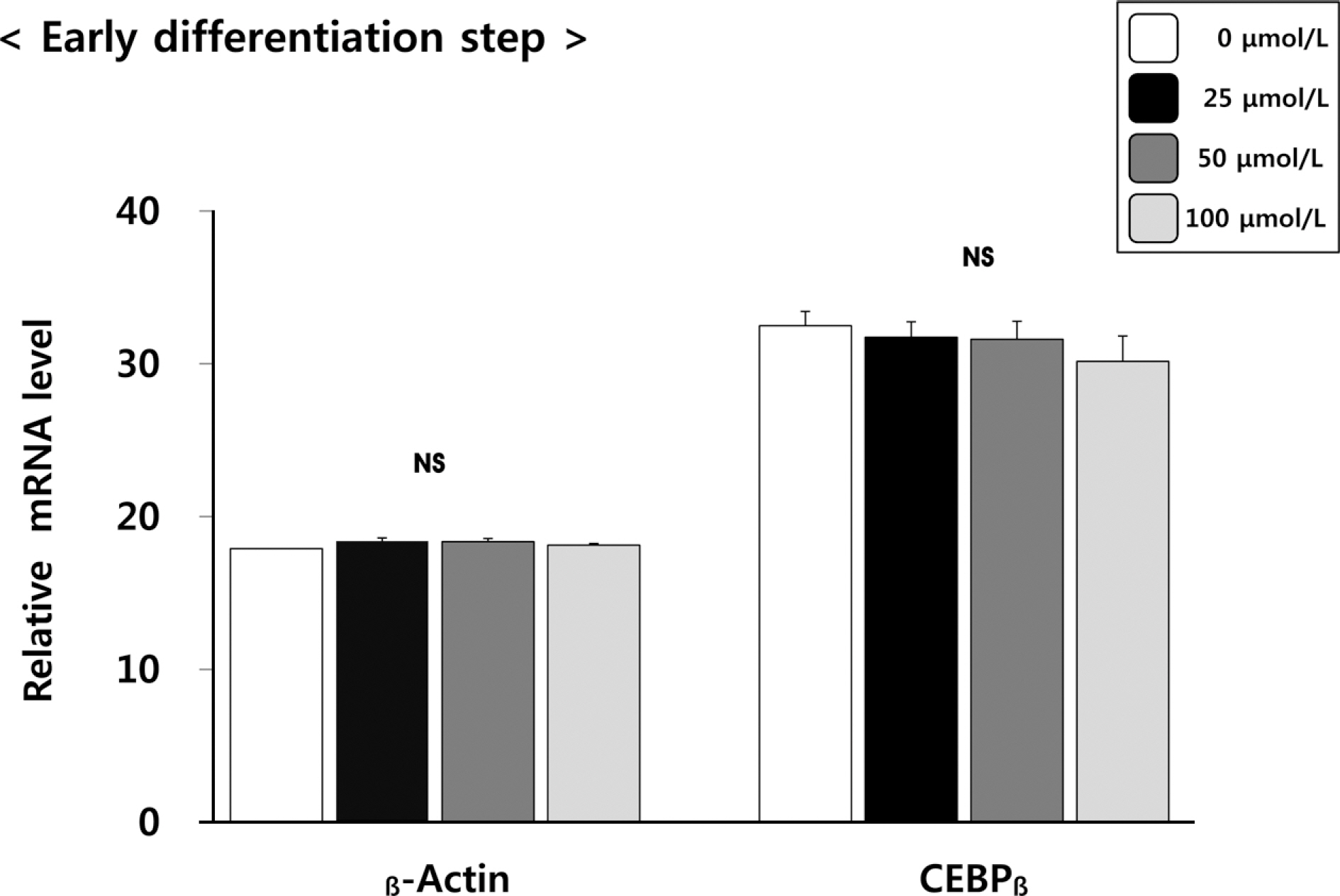
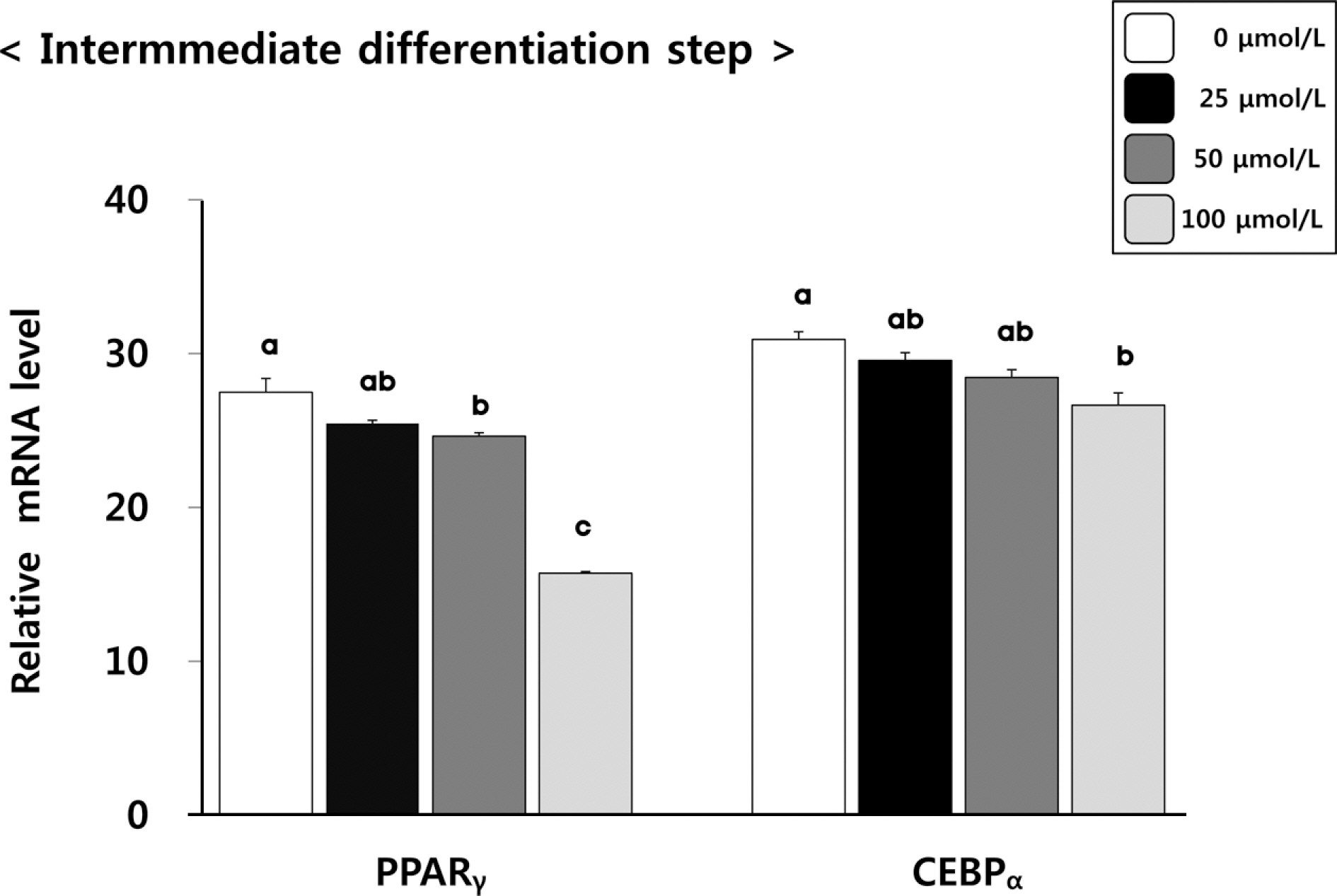
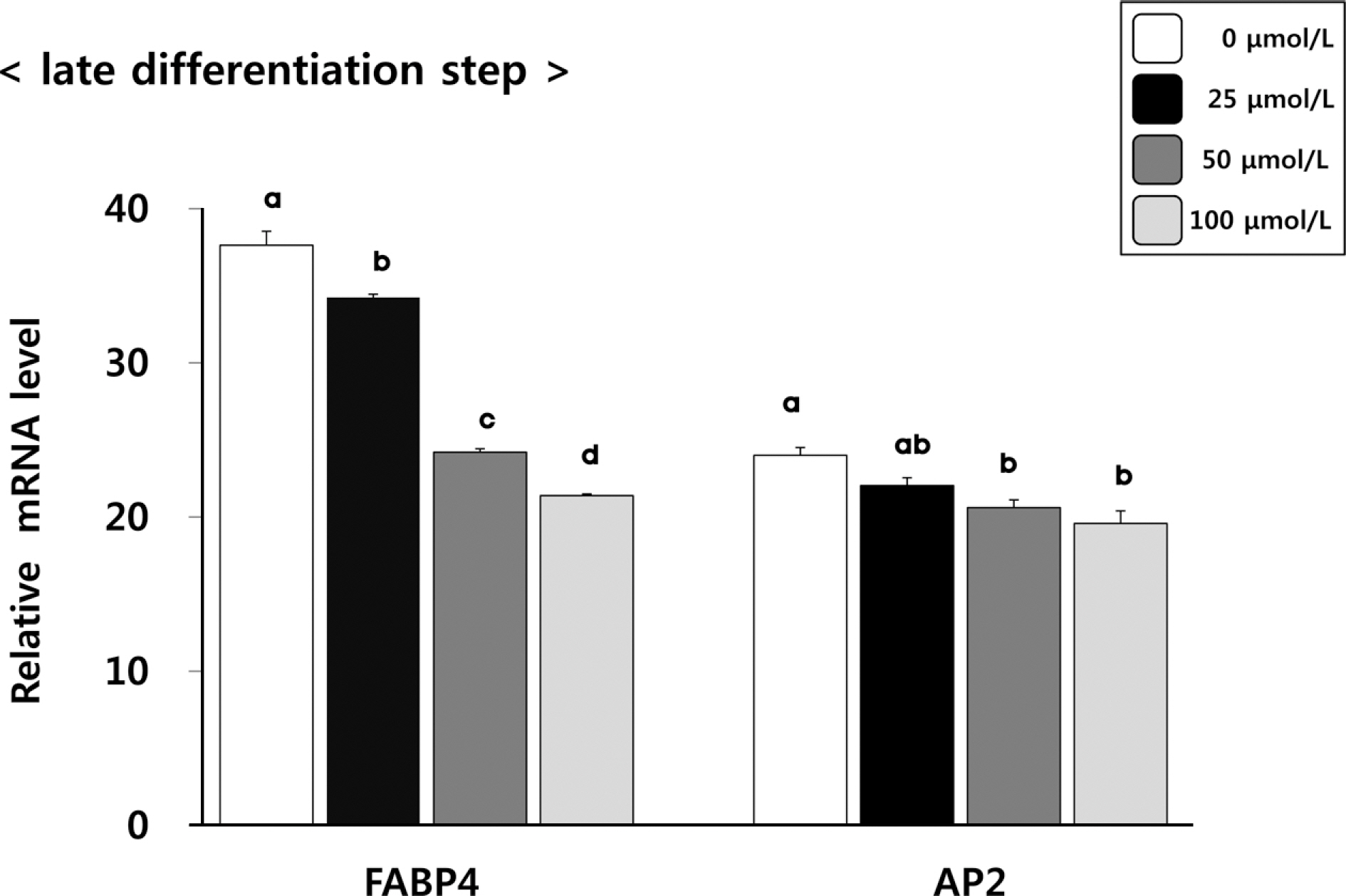
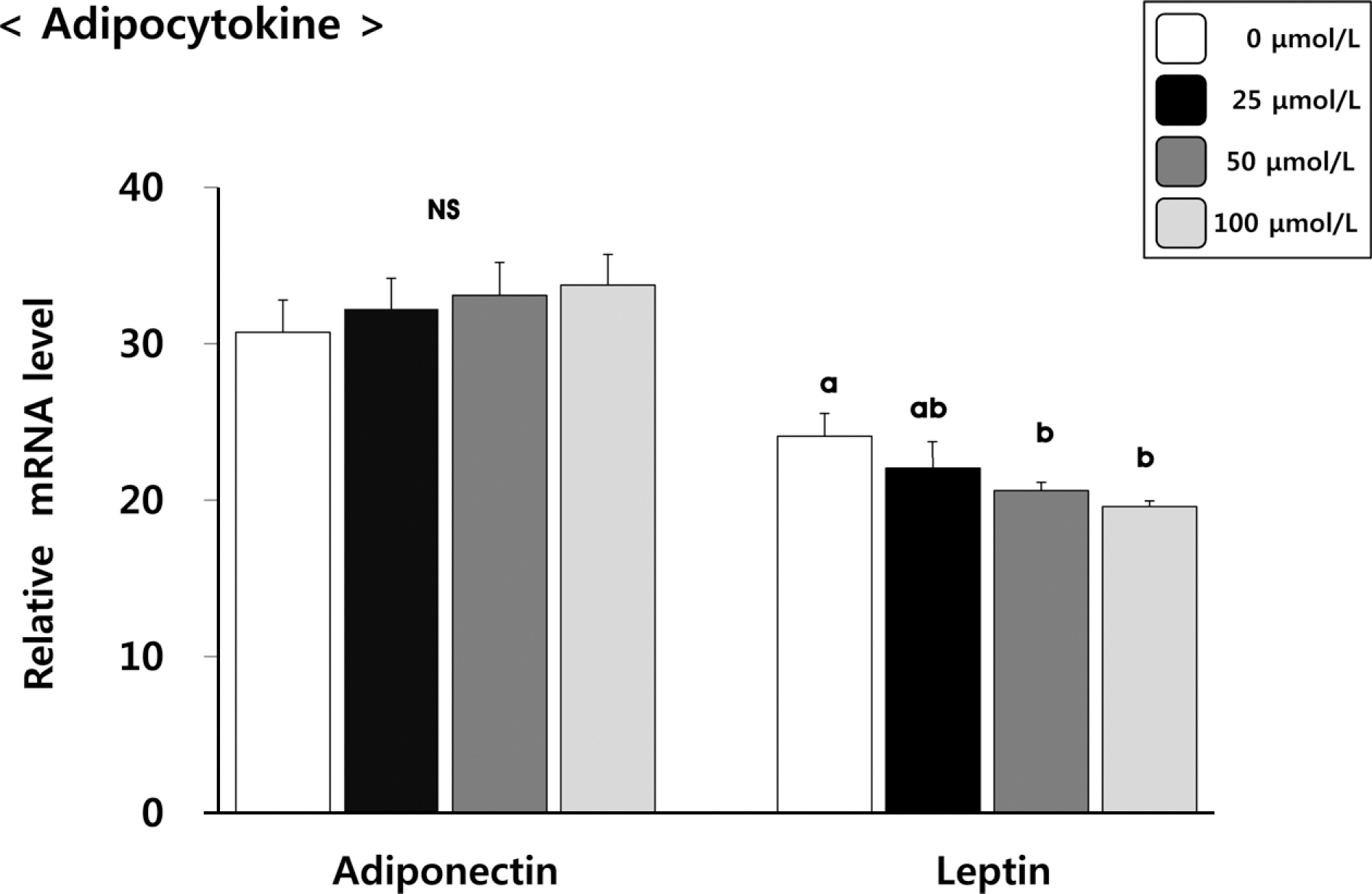
(6)-Gingerol inhibited adipocyte proliferation in a dose and time dependent manner. Expression of C/EBPbeta, associated with early differentiation step remained unchaged. However, intermmediate, late differentiation step and adipocytokines were effectively changed in dose-dependently manner in cell groups treated with (6)-gingerol.
CONCLUSION
This study has shown that treatment with (6)-gingerol inhibited adipocyte proliferation as well as each adipocyte differentiation step. In particular, the (6)-gingerol more effectively inhibited adipocyte differentiation from intermmediate differentiation step.
MeSH Terms
Figure
Reference
-
1.Hirsch J., Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976. 5(2):299–311.
Article2.Harmon AW., Harp JB. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am J Physiol Cell Physiol. 2001. 280(4):C807–C813.
Article3.Ross SR., Graves RA., Greenstein A., Platt KA., Shyu HL., Mellovitz B., Spiegelman BM. A fat-specific enhancer is the primary determinant of gene expression for adipocyte P2 in vivo. Proc Natl Acad Sci U S A. 1990. 87(24):9590–9594.
Article4.Tontonoz P., Hu E., Graves RA., Budavari AI., Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994. 8(10):1224–1234.
Article5.Darlington GJ., Ross SE., MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem. 1998. 273(46):30057–30060.
Article6.Yoshitoshi Y., Naito C., Oda T. The cause and treatment of obesity. Naika. 1964. 13:287–298.7.Noh SK. Functional action of flavonoids for treatment of obesity. Food Ind Nutr. 2002. 7(2):27–29.8.Lee SY. Recent advance in drug therapy of obesity. Proceedings of the Korean Society of Applied Pharmacology Spring Symposium 2001;2001 Apr 13; Kyung Hee University. Seoul: Korean Society of Applied Pharmacology;2001. p. 3–30.9.Beaulieu JF., Quaroni A. Clonal analysis of sucrase-isomaltase expression in the human colon adenocarcinoma Caco-2 cells. Biochem J. 1991. 280(Pt 3):599–608.
Article10.Mathew AG., Krishnamurthy N., Nambudiri ES., Lewis YS. Oil of ginger. Flavour Ind. 1973. 4(5):226–232.11.Kang JH., Ahn BW., Lee DH., Byun HS., Kim SB., Park YH. Inhibitory effects of ginger and garlic extracts on the DNA damage. Korean J Food Sci Technol. 1988. 20(3):287–292.12.Katiyar SK., Agarwal R., Mukhtar H. Inhibition of tumor promotion in SENCAR mouse skin by ethanol extract of Zingiber officinale rhizome. Cancer Res. 1996. 56:1023–1030.13.Shin JH., Lee SJ., Sung NJ. Effects of Zingiber mioga, Zingiber mioga root and Zingiber officinale on the lipid concentration in hyperlipidemic rats. J Korean Soc Food Sci Nutr. 2002. 31(4):679–684.14.Kang NE., Ha AW., Kim JY., Kim WK. Resveratrol inhibits the protein expression of transcription factors related adipocyte differentiation and the activity of matrix metalloproteinase in mouse fibroblast 3T3-L1 preadipocytes. Nutr Res Pract. 2012. 6(6):499–504.
Article15.Kwon SY., Kang KJ. The effect of conjugated linoleic acid isomers on the cell proliferation, apotosis and expressions of uncoupling protein (UCP) genes during differentiation of 3T3-L1 preadipo-cytes. Korean J Nutr. 2004. 37(7):533–539.16.Kim MJ., Yoo YC., Kim HJ., Shin SK., Sohn EJ., Min AY., Sung NY., Kim MR. Aged black garlic exerts anti-inflammatory effects by decreasing no and proinflammatory cytokine production with less cytoxicity in LPS-stimulated raw 264.7 macrophages and LPS-induced septicemia mice. J Med Food. 2014. 17(10):1057–1063.
Article17.Kim WK., Kim JH., Jeong da H., Chun YH., Kim SH., Cho KJ., Chang MJ. Radish (Raphanus sativus L. leaf) ethanol extract inhibits protein and mRNA expression of ErbB(2) and ErbB(3) in MDA-MB-231 human breast cancer cells. Nutr Res Pract. 2011. 5(4):288–293. 18. Chu SK, Seo EY, Kim WK, Kang NE. Effect of cyanidin on cell.18.Chu SK., Seo EY., Kim WK., Kang NE. Effect of cyanidin on cell motility and invasion in MDA-MB-231 human breast cancer cells. Korean J Nutr. 2008. 41(8):711–717.19.Rosen ED., Walkey CJ., Puigserver P., Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000. 14(11):1293–1307.
Article20.Sivakumar V., Sivakumar S. Effect of an indigenous herbal compound preparation ‘Trikatu' on the lipid profiles of atherogenic diet and standard diet fed Rattus norvegicus. Phytother Res. 2004. 18(12):976–981.21.Goyal RK., Kadnur SV. Beneficial effects of Zingiber officinale on goldthioglucose induced obesity. Fitoterapia. 2006. 77(3):160–163.
Article22.Lekstrom-Himes J., Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem. 1998. 273(44):28545–28548.
Article23.Hendricks-Taylor LR., Bachinski LL., Siciliano MJ., Fertitta A., Trask B., de Jong PJ., Ledbetter DH., Darlington GJ. The CCAAT/enhancer binding protein(C/EBP α) gene (CEBPA) maps to human chromosome 19q13.1 and the related nuclear factor NF-IL6 (C/EBP β)gene (CEBPB) maps to human chromosome 20q13.1. Genomics. 1992. 14(1):12–17.24.Zhang DE., Zhang P., Wang ND., Hetherington CJ., Darlington GJ., Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc Natl Acad Sci U S A. 1997. 94(2):569–574.
Article25.Wu Z., Rosen ED., Brun R., Hauser S., Adelmant G., Troy AE., McK-eon C., Darlington GJ., Spiegelman BM. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999. 3(2):151–158.26.Johansen LM., Iwama A., Lodie TA., Sasaki K., Felsher DW., Golub TR., Tenen DG. c-Myc is a critical target for c/EBPα in granulopoiesis. Mol Cell Biol. 2001. 21(11):3789–3806.
Article27.McKnight SL. McBindall—a better name for CCAAT/enhancer binding proteins? Cell. 2001. 107(3):259–261.
Article28.Sugahara K., Iyama KI., Kimura T., Sano K., Darlington GJ., Akiba T., Takiguchi M. Mice lacking CCAAt/enhancer-binding protein-α show hyperproliferation of alveolar type II cells and increased surfactant protein mRNAs. Cell Tissue Res. 2001. 306(1):57–63.
Article29.Takai N., Kawamata N., Walsh CS., Gery S., Desmond JC., Whittaker S., Said JW., Popoviciu LM., Jones PA., Miyakawa I., Koeffler HP. Discovery of epigenetically masked tumor suppressor genes in endometrial cancer. Mol Cancer Res. 2005. 3(5):261–269.
Article30.Tada Y., Brena RM., Hackanson B., Morrison C., Otterson GA., Plass C. Epigenetic modulation of tumor suppressor CCAAT/enhancer binding protein α activity in lung cancer. J Natl Cancer Inst. 2006. 98(6):396–406.
Article31.Nerlov C. C/EBPα mutations in acute myeloid leukaemias. Nat Rev Cancer. 2004. 4(5):394–400.
Article32.Zhu Y., Qi C., Korenberg JR., Chen XN., Noya D., Rao MS., Reddy JK. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPARγ) gene: alternative promoter use and different splicing yield two mPPARγ isoforms. Proc Natl Acad Sci U S A. 1995. 92(17):7921–7925.33.Vidal-Puig A., Jimenez-Liñan M., Lowell BB., Hamann A., Hu E., Spiegelman B., Flier JS., Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996. 97(11):2553–2561.
Article34.Ohnishi R., Matsui-Yuasa I., Deguchi Y., Yaku K., Tabuchi M., Munakata H., Akahoshi Y., Kojima-Yuasa A. 1'-acetoxychavicol acetate inhibits adipogenesis in 3T3-L1 adipocytes and in high fat-fed rats. Am J Chin Med. 2012. 40(6):1189–1204.
Article35.Isa Y., Miyakawa Y., Yanagisawa M., Goto T., Kang MS., Kawada T., Morimitsu Y., Kubota K., Tsuda T. 6-Shogaol and 6-gingerol, the pungent of ginger, inhibit TNF-alpha mediated downregulation of adiponectin expression via different mechanisms in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2008. 373(3):429–434.36.Vidal-Puig A., Jimenez-Liñan M., Lowell BB., Hamann A., Hu E., Spiegelman B., Flier JS., Moller DE. Regulation of PPARγ gene expression by nutrition and obesity in rodents. J Clin Invest. 1996. 97(11):2553–2561.37.Vidal-Puig A., Considine RV., Jimenez-Liñan M., Werman A., Pories WJ., Caro JF., Flier JS. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest. 1997. 99(10):2416–2422.
Article38.Tzeng TF., Chang CJ., Liu IM. 6-gingerol inhibits rosiglitazone-induced adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2014. 28(2):187–192.
Article39.Spiegelman BM., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983. 258(16):10083–10089.
Article40.Hunt CR., Ro JH., Dobson DE., Min HY., Spiegelman BM. Adipocyte P2 gene: developmental expression and homology of 5'-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986. 83(11):3786–3790.
Article41.Hotamisligil GS., Johnson RS., Distel RJ., Ellis R., Papaioannou VE., Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996. 274(5291):1377–1379.42.Uysal KT., Scheja L., Wiesbrock SM., Bonner-Weir S., Hotamisligil GS. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology. 2000. 141(9):3388–3396.
Article43.Tzeng TF., Liu IM. 6-gingerol prevents adipogenesis and the accumulation of cytoplasmic lipid droplets in 3T3-L1 cells. Phytomed-icine. 2013. 20(6):481–487.
Article44.Halaas JL., Gajiwala KS., Maffei M., Cohen SL., Chait BT., Rabinowitz D., Lallone RL., Burley SK., Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995. 269(5223):543–546.45.Tyagi N., Givvimani S., Qipshidze N., Kundu S., Kapoor S., Vacek JC., Tyagi SC. Hydrogen sulfide mitigates matrix metalloprotein-ase-9 activity and neurovascular permeability in hyperhomocyste-inemic mice. Neurochem Int. 2010. 56(2):301–307.
Article46.Saravanan G., Ponmurugan P., Deepa MA., Senthilkumar B. Anti-obesity action of gingerol: effect on lipid profile, insulin, leptin, amylase and lipase in male obese rats induced by a high-fat diet. J Sci Food Agric. 2014. 94(14):2972–2977.
Article47.Okamoto M., Irii H., Tahara Y., Ishii H., Hirao A., Udagawa H., Hira-moto M., Yasuda K., Takanishi A., Shibata S., Shimizu I. Synthesis of a new [6]-gingerol analogue and its protective effect with respect to the development of metabolic syndrome in mice fed a high-fat diet. J Med Chem. 2011. 54(18):6295–6304.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- TonEBP suppresses adipocyte differentiation via modulation of early signaling in 3T3-L1 cells
- The protective effects of steamed ginger on adipogenesis in 3T3-L1 cells and adiposity in diet-induced obese mice
- The Inhibitory Effect of Testosterone on PPARγ-induced Adipogenesis
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3