J Korean Soc Transplant.
2014 Jun;28(2):83-86. 10.4285/jkstn.2014.28.2.83.
A Case of Squamous Cell Carcinoma in Nasal Cavity Treated with Conversion to Sirolimus in a Patient with Kidney Transplantation
- Affiliations
-
- 1Department of Internal Medicine, Bong Seng Memorial Hospital, Busan, Korea. kidney119@hotmail.com
- KMID: 2202535
- DOI: http://doi.org/10.4285/jkstn.2014.28.2.83
Abstract
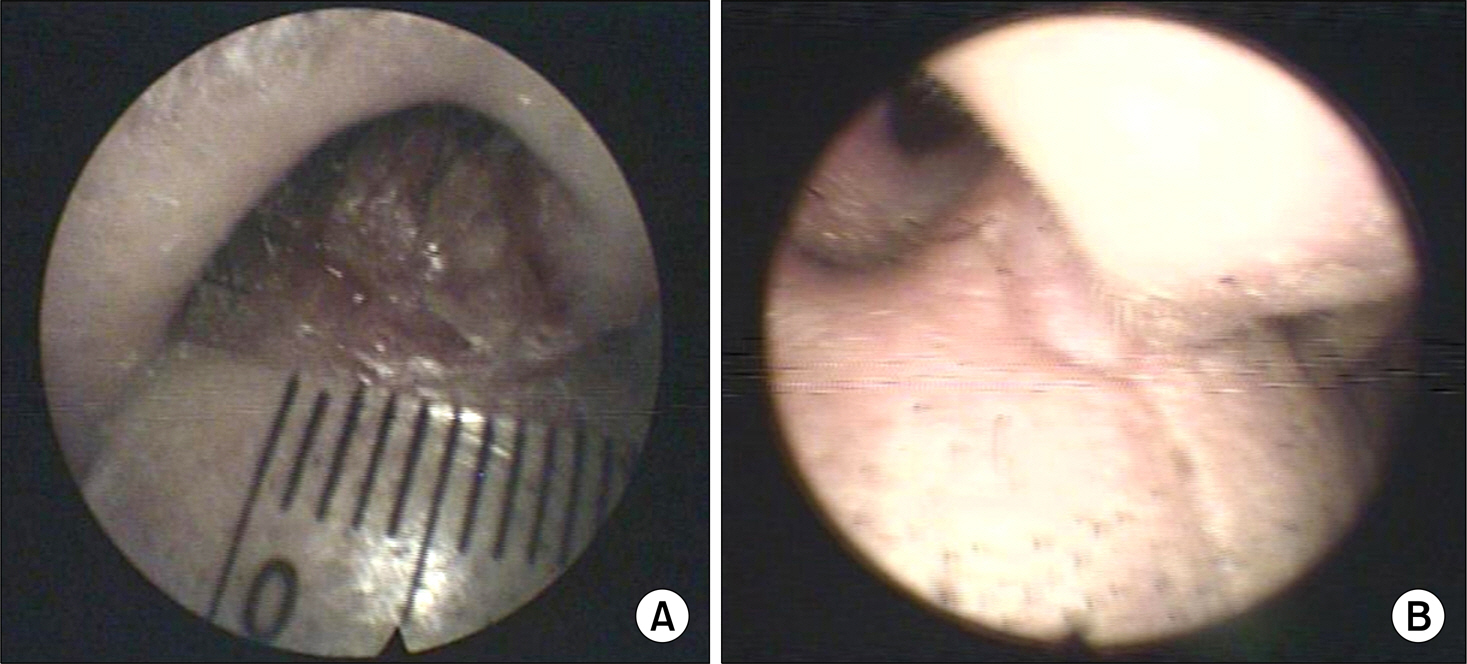
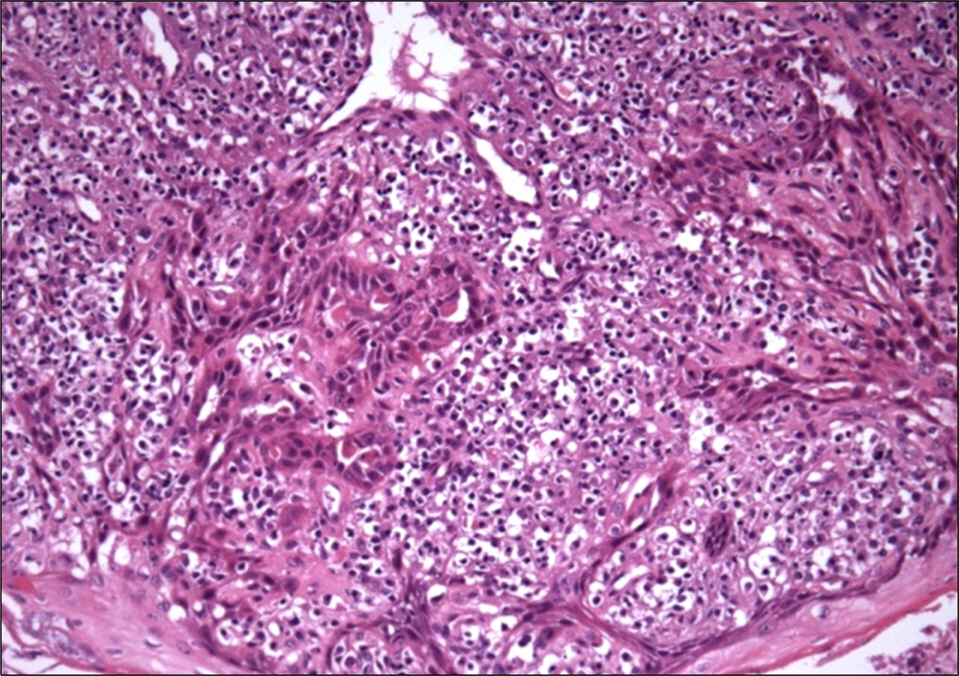
- Conversion of immunosuppressants to sirolimus, an inhibitor of mammalian target of rapamycin, is a useful treatment option for prevention of the adverse events of immunosuppressants such as calcineurin inhibitor in renal transplantation recipients. In addition, sirolimus has been improving the quality of life and increasing the survival of patients with renal transplantation by decreasing immunosuppression-related malignancies, particularly skin cancer. However, complete remission of skin squamous cell carcinoma after renal transplantation only by conversion to sirolimus has not been well reported, although its preventive effect on skin cancer is well known. We report on a 72-year-old male with squamous cell carcinoma in his nasal cavity consequent to renal transplantation, which was treated completely with the conversion of cyclosporine to sirolimus without surgical removal or chemotherapy.
MeSH Terms
Figure
Reference
-
References
1). Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003; 348:1681–91.
Article2). Ulrich C, Schmook T, Sachse MM, Sterry W, Stockfleth E. Comparative epidemiology and pathogenic factors for nonmelanoma skin cancer in organ transplant patients. Dermatol Surg. 2004; 30(4 Pt 2):622–7.
Article3). Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part I. Epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011; 65:253–61.4). Stasko T, Brown MD, Carucci JA, Euvrard S, Johnson TM, Sengelmann RD, et al. Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Dermatol Surg. 2004; 30(4 Pt 2):642–50.
Article5). EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.6.1. Cancer risk after renal transplantation. Posttransplant lymphoproliferative disease (PTLD): pre-vention and treatment. Nephrol Dial Transplant. 2002; 17(Suppl 4):31–6.6). Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011; 65:263–79.7). Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004; 18:446–9.
Article8). Salgo R, Gossmann J, Schofer H, Kachel HG, Kuck J, Geiger H, et al. Switch to a sirolimus-based immunosuppression in longterm renal transplant recipients: reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant. 2010; 10:1385–93.
Article9). Ho WL, Murphy GM. Update on the pathogenesis of post-transplant skin cancer in renal transplant recipients. Br J Dermatol. 2008; 158:217–24.
Article10). Bouwes Bavinck JN, Hardie DR, Green A, Cutmore S, MacNaught A, O'Sullivan B, et al. The risk of skin cancer in renal transplant recipients in Queensland, Australia. A follow-up study. Transplantation. 1996; 61:715–21.11). Ducloux D, Carron PL, Rebibou JM, Aubin F, Fournier V, Bresson-Vautrin C, et al. CD4 lymphocytopenia as a risk factor for skin cancers in renal transplant recipients. Transplantation. 1998; 65:1270–2.
Article12). O'Donovan P, Perrett CM, Zhang X, Montaner B, Xu YZ, Harwood CA, et al. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005; 309:1871–4.13). Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005; 80(2 Suppl):S254–64.
Article14). Robson R, Cecka JM, Opelz G, Budde M, Sacks S. Prospective registry-based observational cohort study of the longterm risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant. 2005; 5:2954–60.
Article15). Baczkowska T, Perkowska-Ptasiń ska A, Sadowska A, Lewandowski Z, Nowacka-Cieciura E, Cieciura T, et al. Serum TGF-beta1 correlates with chronic histopathological lesions in protocol biopsies of kidney allograft recipients. Transplant Proc. 2005; 37:773–5.16). Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003; 35(3 Suppl):7S–14S.
Article17). Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002; 8:128–35.
Article18). Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012; 367:329–39.
Article19). Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, Proby CM, Wolterbeek R, Bouwes Bavinck JN, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013; 31:1317–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Basaloid Squamous Cell Carcinoma in the Nasal Cavity
- Verruca Eradication Following Conversion to Sirolimus in a Renal Transplant Recipient after Longstanding Cyclosporine A Treatment
- Hybrid verrucous squamous cell carcinoma of sinonasal tract: a case report
- A Case of Verrucous Carcinoma of the Hard Palate and the Nasal Floor
- A Case of Squamous Cell Carcinoma of Nasal Septum Removed Through the External Rhinoplasty Approach