J Lung Cancer.
2011 Jun;10(1):13-25. 10.6058/jlc.2011.10.1.13.
Circulating Tumor Cells in Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Wonkwang University School of Medicine, Iksan, Korea. yshpul@wonkwang.ac.kr
- 2Genome Research Center for Immune Disorders, Wonkwang University School of Medicine, Iksan, Korea.
- 3Department of Ophthalmology, Wonkwang University School of Medicine, Iksan, Korea.
- KMID: 2199890
- DOI: http://doi.org/10.6058/jlc.2011.10.1.13
Abstract
- Circulating Tumour Cells (CTCs) can be released from the primary lung tumour into the bloodstream and they may colonize distant organs and give rise to metastasis. The presence of CTCs in the blood has been documented more than a century ago, and ultrasensitive methods have been recently developed to detect circulating tumour cells (CTCs) in the peripheral blood of lung cancer patients. Most CTCs require an initial enrichment step, since CTCs are a very rare event. The different technologies and also the differences among the screened populations make the clinical significance of detecting CTCs difficult to interpret. Peripheral blood analyses are more convenient for patients than invasive BM sampling and many research groups are currently assessing the clinical utility of CTCs for assessing the prognosis and monitoring the response to systemic therapies in lung cancer patients. Here we will review the different assays that are currently available for CTC detection and analysis of lung cancer. Moreover, molecular analyses of CTCs have provided new insights into the biology of metastasis of lung cancer with important implications for the clinical management of lung cancer patients.
MeSH Terms
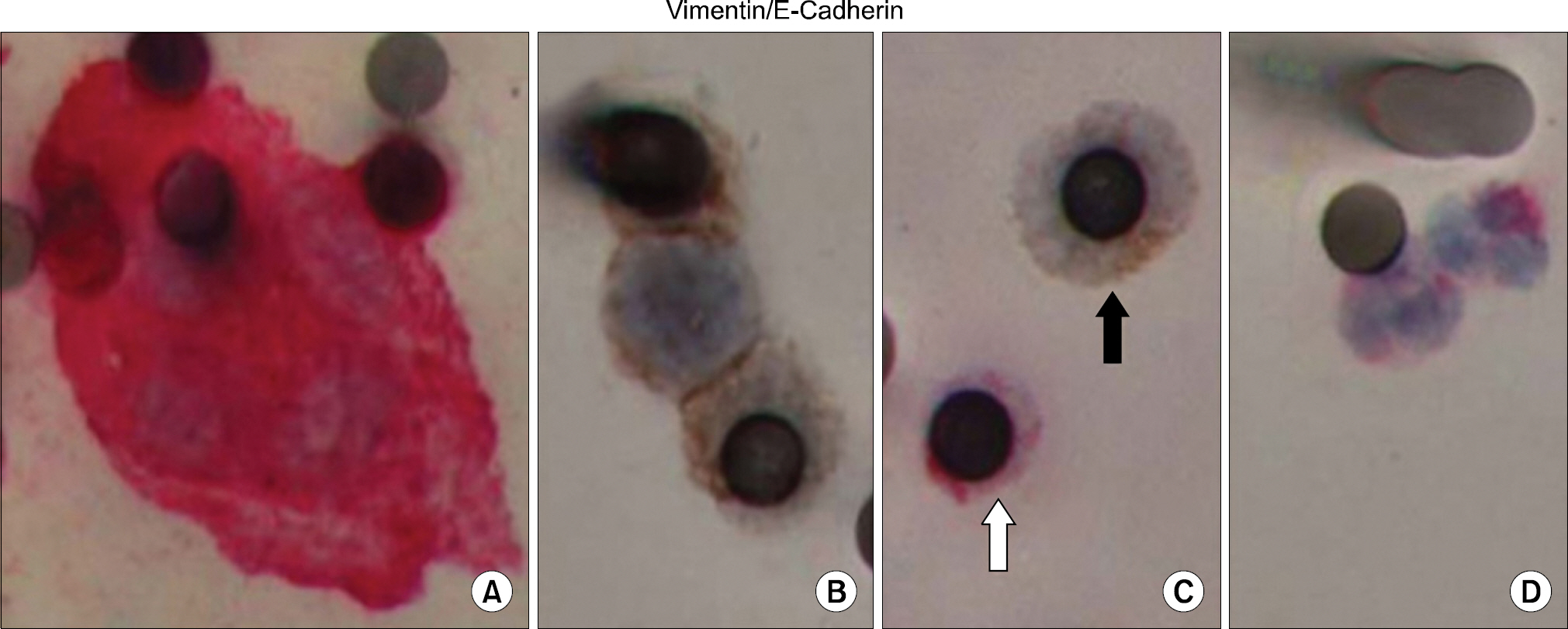
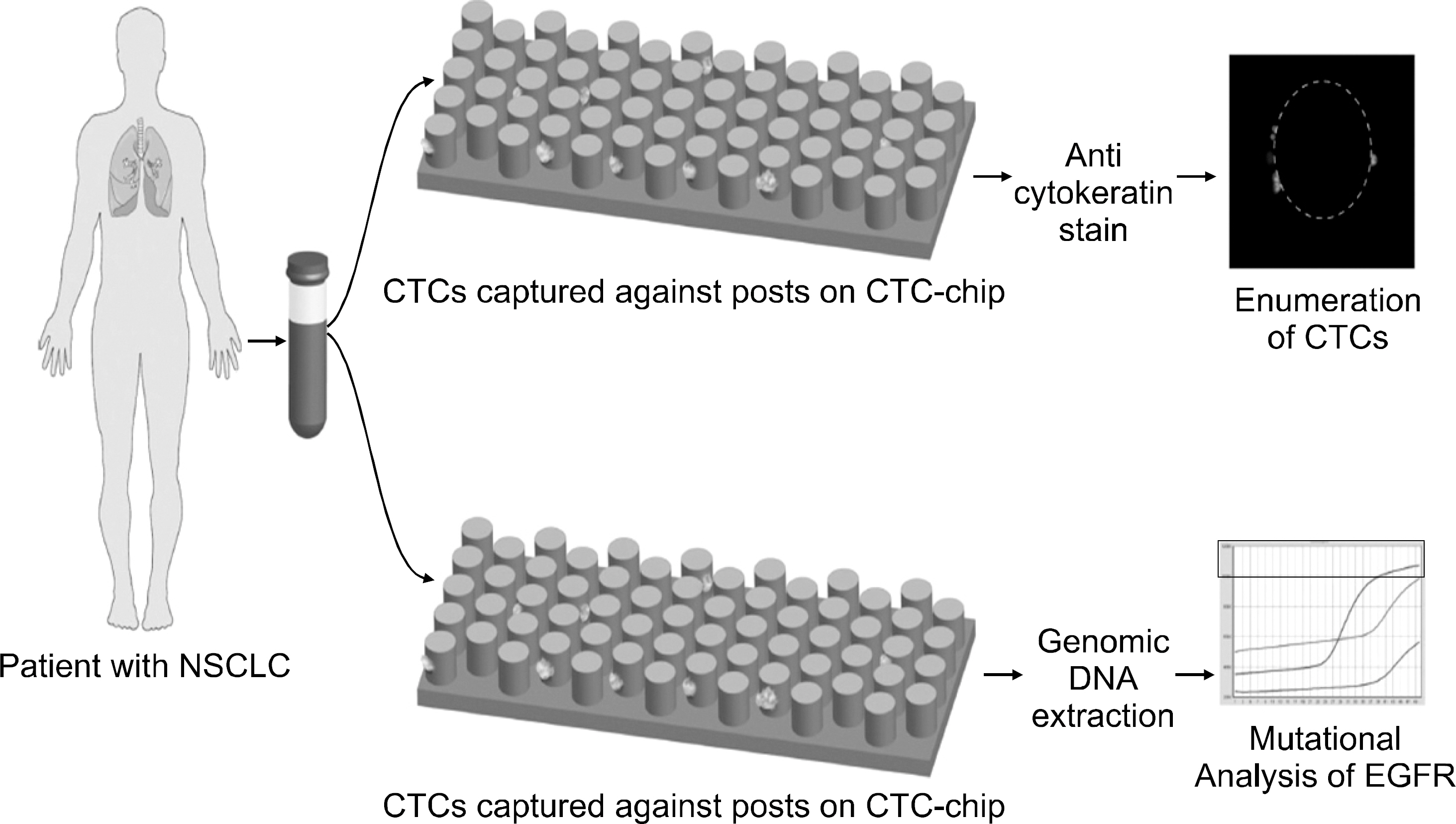
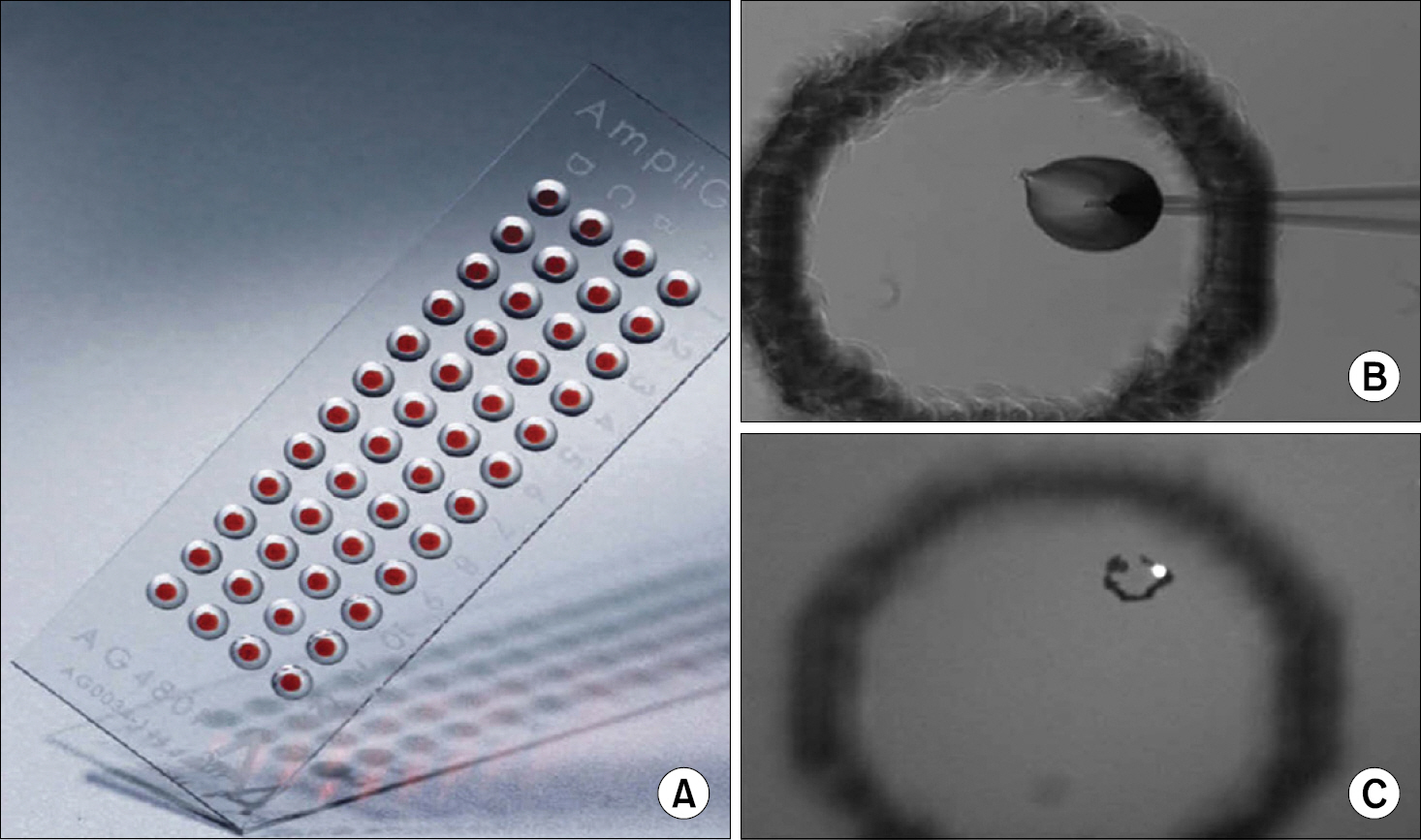
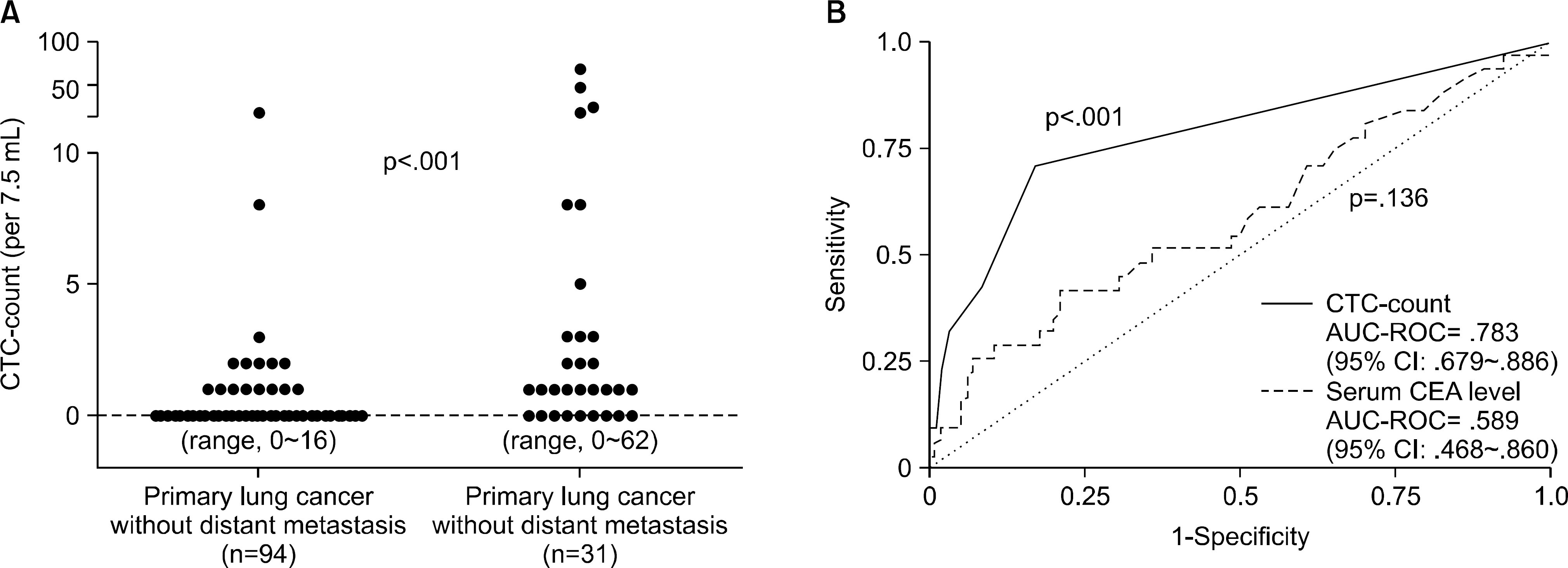
Figure
Reference
-
1. Socinski MA, Crowell R, Hensing TE, et al. Treatment of non-small cell lung cancer, stage IV: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007; 132(3 Suppl):277S–289S.2. Alix-Panabiè res C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res. 2008; 14:5013–5021.
Article3. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989; 8:98–101.4. Fidler IJ. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978; 38:2651–2660.5. Riethdorf S, Wikman H, Pantel K. Review: biological relevance of disseminated tumor cells in cancer patients. Int J Cancer. 2008; 123:1991–2006.
Article6. Bernards R, Weinberg RA. A progression puzzle. Nature. 2002; 418:823.7. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009; 119:1420–1428.
Article8. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002; 2:442–454.
Article9. Ashworth TR. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aust Med J. 1869; 14:146–147.10. Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomor-phological and molecular characterization of circulatingtumor cells. Am J Pathol. 2000; 156:57–63.11. Gertler R, Rosenberg R, Fuehrer K, Dahm M, Nekarda H, Siewert JR. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. Recent Results Cancer Res. 2003; 162:149–155.
Article12. Mü ller V, Stahmann N, Riethdorf S, et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res. 2005; 11:3678–3685.
Article13. Naume B, Borgen E, T⊘ ssvik S, Pavlak N, Oates D, Nesland JM. Detection of isolated tumor cells in peripheral blood and in BM: evaluation of a new enrichment method. Cytotherapy. 2004; 6:244–252.
Article14. Hayes GM, Busch R, Voogt J, et al. Isolation of malignant B cells from patients with chronic lymphocytic leukemia (CLL) for analysis of cell proliferation: validation of a simplified method suitable for multi-center clinical studies. Leuk Res. 2010; 34:809–815.
Article15. Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004; 10:6897–6904.
Article16. Mü ller V, Alix-Panabiè res C, Pantel K. Insights into minimal residual disease in cancer patients: implications for anti-cancer therapies. Eur J Cancer. 2010; 46:1189–1197.17. Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011; 178:989–996.
Article18. Smith BM, Slade MJ, English J, et al. Response of circulating tumor cells to systemic therapy in patients with metastatic breast cancer: comparison of quantitative polymerase chain reaction and immunocytochemical techniques. J Clin Oncol. 2000; 18:1432–1439.
Article19. Lambrechts AC, Bosma AJ, Klaver SG, et al. Comparison of immunocytochemistry, reverse transcriptase polymerase chain reaction, and nucleic acid sequence-based amplification for the detection of circulating breast cancer cells. Breast Cancer Res Treat. 1999; 56:219–231.
Article20. Krivacic RT, Ladanyi A, Curry DN, et al. A rare-cell detector for cancer. Proc Natl Acad Sci USA. 2004; 101:10501–10504.
Article21. Cruz I, Ciudad J, Cruz JJ, et al. Evaluation of multiparameter flow cytometry for the detection of breast cancer tumor cells in blood samples. Am J Clin Pathol. 2005; 123:66–64.
Article22. Simpson SJ, Vachula M, Kennedy MJ, et al. Detection of tumor cells in the bone marrow, peripheral blood, and apheresis products of breast cancer patients using flow cytometry. Exp Hematol. 1995; 23:1062–1068.23. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004; 351:781–791.
Article24. Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005; 23:1420–1430.
Article25. Danila DC, Heller G, Gignac GA, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007; 13:7053–7058.
Article26. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008; 26:3213–3221.
Article27. Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007; 450:1235–1239.
Article28. Sequist LV, Nagrath S, Toner M, Haber DA, Lynch TJ. The CTC-chip: an exciting new tool to detect circulating tumor cells in lung cancer patients. J Thorac Oncol. 2009; 4:281–283.
Article29. Alix-Panabiè res C, Vendrell JP, Pellé O, et al. Detection and characterization of putative metastatic precursor cells in cancer patients. Clin Chem. 2007; 53:537–539.30. Alix-Panabiè res C, Brouillet JP, Fabbro M, et al. Charac-terization and enumeration of cells secreting tumor markers in the peripheral blood of breast cancer patients. J Immunol Methods. 2005; 299:177–188.31. Holodick NE, Repetny K, Zhong X, Rothstein TL. Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur J Immunol. 2009; 39:2383–2394.
Article32. Waak J, Weber SS, Waldenmaier A, et al. Regulation of astrocyte inflammatory responses by the Parkinson's disease- associated gene DJ-1. FASEB J. 2009; 23:2478–2489.33. Meng S, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004; 10:8152–8162.
Article34. Meng S, Tripathy D, Shete S, et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci USA. 2004; 101:9393–9398.
Article35. Schmidt-Kittler O, Ragg T, Daskalakis A, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci USA. 2003; 100:7737–7742.
Article36. Tanaka F, Yoneda K, Kondo N, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res. 2009; 15:6980–6986.
Article37. Yoon SO, Kim YT, Jung KC, Jeon YK, Kim BH, Kim CW. TTF-1 mRNA-positive circulating tumor cells in the peripheral blood predict poor prognosis in surgically resected non-small cell lung cancer patients. Lung Cancer. 2011; 71:209–216.
Article38. Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008; 359:366–377.39. Wu C, Hao H, Li L, et al. Preliminary investigation of the clinical significance of detecting circulating tumor cells enriched from lung cancer patients. J Thorac Oncol. 2009; 4:30–36.
Article40. Pantel K, Schlimok G, Braun S, et al. Differential expression of proliferation-associated molecules in individual micrometa-static carcinoma cells. J Natl Cancer Inst. 1993; 85:1419–1424.
Article41. Pantel K, Izbicki JR, Angstwurm M, et al. Immunocytological detection of bone marrow micrometastasis in operable non-small cell lung cancer. Cancer Res. 1993; 53:1027–1031.42. Pantel K, Alix-Panabieres C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010; 16:389–406.
Article43. Alunni-Fabbroni M, Sandri MT. Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods. 2010; 50:289–297.
Article44. Mostert B, Sleijfer S, Foekens JA, et al. Circulating tumor cells (CTCs): detection methods and their clinical relevance in breast cancer. Cancer Treat Rev. 2009; 35:463–474.
Article45. Nolé F, Munzone E, Zorzino L, et al. Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol. 2008; 19:891–897.
Article46. Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006; 12:6403–6409.
Article47. De Giorgi U, Valero V, Rohren E, et al. Circulating tumor cells and [18F]fluorodeoxyglucose positron emission tomography/computed tomography for outcome prediction in metastatic breast cancer. J Clin Oncol. 2009; 10:3303–3311.
Article48. Pierga JY, Bidard FC, Mathiot C, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008; 14:7004–7010.
Article49. Stathopoulou A, Vlachonikolis I, Mavroudis D, et al. Molecular detection of cytokeratin 19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol. 2002; 20:3404–3412.50. Xenidis N, Perraki M, Kafousi M, et al. Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA- positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients. J Clin Oncol. 2006; 24:3756–3762.51. Ignatiadis M, Xenidis N, Perraki M, et al. Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol. 2007; 25:5194–5202.52. Apostolaki S, Perraki M, Pallis A, et al. Circulating HER2 mRNA-positive cells in the peripheral blood of patients with stage I and II breast cancer after the administration of adjuvant chemotherapy: evaluation of their clinical relevance. Ann Oncol. 2007; 18:851–858.
Article53. Xenidis N, Ignatiadis M, Apostolaki S, et al. Cytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancer. J Clin Oncol. 2009; 27:2177–2184.
Article54. Rack B, Schindlbeck C, Jü ckstock J, et al. Prevalence of CA 27.29 in primary breast cancer patients before the start of systemic treatment. Anticancer Res. 2010; 30:1837–1841.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Circulating Tumor Cell and Cell-free Circulating Tumor DNA in Lung Cancer
- Circulating Tumor Cells and Extracellular Nucleic Acids in Breast Cancer
- Circulating Tumor Cells: Detection Methods and Potential Clinical Application in Breast Cancer
- Clinical Application of Circulating Tumor DNA Analysis
- Circulating Aneuploid Cells Detected in the Blood of Patients with Infectious Lung Diseases