J Clin Neurol.
2011 Dec;7(4):173-183. 10.3988/jcn.2011.7.4.173.
Treatment of Myasthenia Gravis Based on Its Immunopathogenesis
- Affiliations
-
- 1Department of Neurology, Kwandong University College of Medicine, Myongji Hospital, Goyang, Korea.
- 2Department of Neurology, Ewha Womans University School of Medicine, Seoul, Korea.
- 3Department of Neurology, University of California School of Medicine, Davis, CA, USA. dprichman@ucdavis.edu
- KMID: 2179002
- DOI: http://doi.org/10.3988/jcn.2011.7.4.173
Abstract
- The prognosis of myasthenia gravis (MG) has improved dramatically due to advances in critical-care medicine and symptomatic treatments. Its immunopathogenesis is fundamentally a T-cell-dependent autoimmune process resulting from loss of tolerance toward self-antigens in the thymus. Thymectomy is based on this immunological background. For MG patients who are inadequately controlled with sufficient symptomatic treatment or fail to achieve remission after thymectomy, remission is usually achieved through the addition of other immunotherapies. These immunotherapies can be classified into two groups: rapid induction and long-term maintenance. Rapid induction therapy includes intravenous immunoglobulin (IVIg) and plasma exchange (PE). These produce improvement within a few days after initiation, and so are useful for acute exacerbation including myasthenic crisis or in the perioperative period. High-dose prednisone has been more universally preferred for remission induction, but it acts more slowly than IVIg and PE, commonly only after a delay of several weeks. Slow tapering of steroids after a high-dose pulse offers a method of maintaining the state of remission. However, because of significant side effects, other immunosuppressants (ISs) are frequently added as "steroid-sparing agents". The currently available ISs exert their immunosuppressive effects by three mechanisms: 1) blocking the synthesis of DNA and RNA, 2) inhibiting T-cell activation and 3) depleting the B-cell population. In addition, newer drugs including antisense molecule, tumor necrosis factor alpha receptor blocker and complement inhibitors are currently under investigation to confirm their effectiveness. Until now, the treatment of MG has been based primarily on experience rather than gold-standard evidence from randomized controlled trials. It is hoped that well-organized studies and newer experimental trials will lead to improved treatments.
MeSH Terms
-
Autoantigens
B-Lymphocytes
Complement System Proteins
DNA
Humans
Immunoglobulins
Immunoglobulins, Intravenous
Immunosuppressive Agents
Immunotherapy
Myasthenia Gravis
Perioperative Period
Plasma Exchange
Prednisone
Prognosis
Remission Induction
RNA
Steroids
T-Lymphocytes
Thymectomy
Thymus Gland
Tumor Necrosis Factor-alpha
Autoantigens
Complement System Proteins
DNA
Immunoglobulins
Immunoglobulins, Intravenous
Immunosuppressive Agents
Prednisone
RNA
Steroids
Tumor Necrosis Factor-alpha
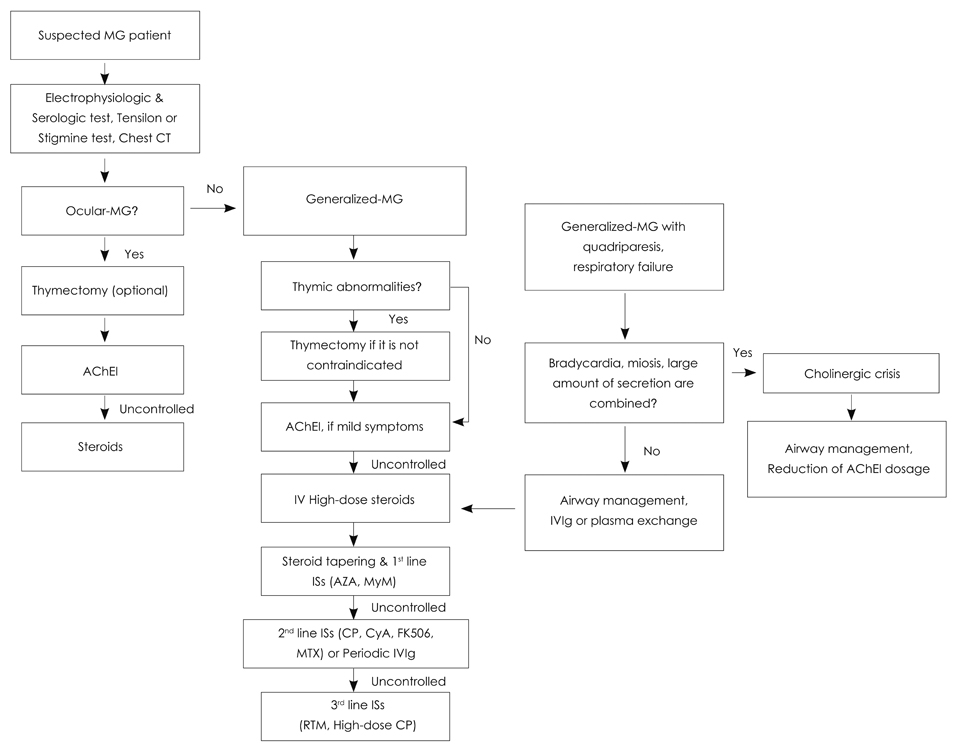
Figure
Reference
-
1. Sanders DB, Howard JF Jr. Bradely WG, Daroff RB, Fenichel GM, Jankovic J, editors. Disorders of neuromuscular transmission. Neurology in Clinical Practice. 2004. 4th ed. Philadelphia: Butterworth-Heinemann;2441–2462.
Article2. Richman DP, Agius MA. Treatment of autoimmune myasthenia gravis. Neurology. 2003. 61:1652–1661.
Article3. Drachman DB. Myasthenia gravis. N Engl J Med. 1994. 330:1797–1810.
Article4. Dalakas MC. Intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: present status and practical therapeutic guidelines. Muscle Nerve. 1999. 22:1479–1497.
Article5. Gajdos P, Chevret S, Toyka K. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database Syst Rev. 2008. CD002277.
Article6. Wolfe GI, Barohn RJ, Foster BM, Jackson CE, Kissel JT, Day JW, et al. Randomized, controlled trial of intravenous immunoglobulin in myasthenia gravis. Muscle Nerve. 2002. 26:549–552.
Article7. Zinman L, Ng E, Bril V. IV immunoglobulin in patients with myasthenia gravis: a randomized controlled trial. Neurology. 2007. 68:837–841.
Article8. Gajdos P, Chevret S, Clair B, Tranchant C, Chastang C. Myasthenia Gravis Clinical Study Group. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Ann Neurol. 1997. 41:789–796.
Article9. Rønager J, Ravnborg M, Hermansen I, Vorstrup S. Immunoglobulin treatment versus plasma exchange in patients with chronic moderate to severe myasthenia gravis. Artif Organs. 2001. 25:967–973.
Article10. Gajdos P, Tranchant C, Clair B, Bolgert F, Eymard B, Stojkovic T, et al. Treatment of myasthenia gravis exacerbation with intravenous immunoglobulin: a randomized double-blind clinical trial. Arch Neurol. 2005. 62:1689–1693.
Article11. Assessment of plasmapheresis. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996. 47:840–843.12. Batocchi AP, Evoli A, Di Schino C, Tonali P. Therapeutic apheresis in myasthenia gravis. Ther Apher. 2000. 4:275–279.13. Yeh JH, Chiu HC. Comparison between double-filtration plasmapheresis and immunoadsorption plasmapheresis in the treatment of patients with myasthenia gravis. J Neurol. 2000. 247:510–513.
Article14. Liu JF, Wang WX, Xue J, Zhao CB, You HZ, Lu JH, et al. Comparing the autoantibody levels and clinical efficacy of double filtration plasmapheresis, immunoadsorption, and intravenous immunoglobulin for the treatment of late-onset myasthenia gravis. Ther Apher Dial. 2010. 14:153–160.
Article15. Blalock A, Mason MF, Morgan HJ, Riven SS. Myasthenia gravis and tumors of the thymic region: report of a case in which the tumor was removed. Ann Surg. 1939. 110:544–561.16. Hennessey IA, Long AM, Hughes I, Humphrey G. Thymectomy for inducing remission in juvenile myasthenia gravis. Pediatr Surg Int. 2011. 27:591–594.
Article17. Brearley S, Gentle TA, Baynham MI, Roberts KD, Abrams LD, Thompson RA. Immunodeficiency following neonatal thymectomy in man. Clin Exp Immunol. 1987. 70:322–327.18. Ponseti JM, Caritg N, Gamez J, López-Cano M, Vilallonga R, Armengol M. A comparison of long-term post-thymectomy outcome of anti-AChR-positive, anti-AChR-negative and anti-MuSK-positive patients with non-thymomatous myasthenia gravis. Expert Opin Biol Ther. 2009. 9:1–8.
Article19. Lauriola L, Ranelletti F, Maggiano N, Guerriero M, Punzi C, Marsili F, et al. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology. 2005. 64:536–538.
Article20. Maggi G, Casadio C, Cavallo A, Cianci R, Molinatti M, Ruffini E. Thymectomy in myasthenia gravis. Results of 662 cases operated upon in 15 years. Eur J Cardiothorac Surg. 1989. 3:504–509. discussion 510-511.21. Zielinski M, Hauer L, Hauer J, Pankowski J, Nabialek T, Szlubowski A. Comparison of complete remission rates after 5 year follow-up of three different techniques of thymectomy for myasthenia gravis. Eur J Cardiothorac Surg. 2010. 37:1137–1143.
Article22. Meyer DM, Herbert MA, Sobhani NC, Tavakolian P, Duncan A, Bruns M, et al. Comparative clinical outcomes of thymectomy for myasthenia gravis performed by extended transsternal and minimally invasive approaches. Ann Thorac Surg. 2009. 87:385–390. discussion 390-391.
Article23. Díaz-Manera J, Rojas-García R, Illa I. Treatment strategies for myasthenia gravis. Expert Opin Pharmacother. 2009. 10:1329–1342.
Article24. Schneider-Gold C, Gajdos P, Toyka KV, Hohlfeld RR. Corticosteroids for myasthenia gravis. Cochrane Database Syst Rev. 2005. CD002828.
Article25. Mount FW. Acth for ocular myasthenia. JAMA. 1964. 189:55.
Article26. Howard FM Jr, Duane DD, Lambert EH, Daube JR. Alternate-day prednisone: preliminary report of a double-blind controlled study. Ann N Y Acad Sci. 1976. 274:596–607.
Article27. Lindberg C, Andersen O, Lefvert AK. Treatment of myasthenia gravis with methylprednisolone pulse: a double blind study. Acta Neurol Scand. 1998. 97:370–373.
Article28. Wegner B, Ahmed I. Intravenous immunoglobulin monotherapy in long-term treatment of myasthenia gravis. Clin Neurol Neurosurg. 2002. 105:3–8.
Article29. Witte AS, Cornblath DR, Parry GJ, Lisak RP, Schatz NJ. Azathioprine in the treatment of myasthenia gravis. Ann Neurol. 1984. 15:602–605.
Article30. Mantegazza R, Antozzi C, Peluchetti D, Sghirlanzoni A, Cornelio F. Azathioprine as a single drug or in combination with steroids in the treatment of myasthenia gravis. J Neurol. 1988. 235:449–453.
Article31. Palace J, Newsom-Davis J, Lecky B. Myasthenia Gravis Study Group. A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Neurology. 1998. 50:1778–1783.
Article32. Myasthenia Gravis Clinical Study Group. A randomised clinical trial comparing prednisone and azathioprine in myasthenia gravis. Results of the second interim analysis. J Neurol Neurosurg Psychiatry. 1993. 56:1157–1163.33. Sanders DB, Evoli A. Immunosuppressive therapies in myasthenia gravis. Autoimmunity. 2010. 43:428–435.
Article34. Ciafaloni E, Massey JM, Tucker-Lipscomb B, Sanders DB. Mycophenolate mofetil for myasthenia gravis: an open-label pilot study. Neurology. 2001. 56:97–99.
Article35. Meriggioli MN, Rowin J, Richman JG, Leurgans S. Mycophenolate mofetil for myasthenia gravis: a double-blind, placebo-controlled pilot study. Ann N Y Acad Sci. 2003. 998:494–499.
Article36. Muscle Study Group. A trial of mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology. 2008. 71:394–399.37. Sanders DB, Hart IK, Mantegazza R, Shukla SS, Siddiqi ZA, De Baets MH, et al. An international, phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology. 2008. 71:400–406.
Article38. Hehir MK, Burns TM, Alpers J, Conaway MR, Sawa M, Sanders DB. Mycophenolate mofetil in AChR-antibody-positive myasthenia gravis: outcomes in 102 patients. Muscle Nerve. 2010. 41:593–598.
Article39. Engelen W, Verpooten GA, Van der Planken M, Helbert MF, Bosmans JL, De Broe ME. Four cases of red blood cell aplasia in association with the use of mycophenolate mofetil in renal transplant patients. Clin Nephrol. 2003. 60:119–124.
Article40. Neff RT, Hurst FP, Falta EM, Bohen EM, Lentine KL, Dharnidharka VR, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation. 2008. 86:1474–1478.
Article41. Perez MC, Buot WL, Mercado-Danguilan C, Bagabaldo ZG, Renales LD. Stable remissions in myasthenia gravis. Neurology. 1981. 31:32–37.42. De Feo LG, Schottlender J, Martelli NA, Molfino NA. Use of intravenous pulsed cyclophosphamide in severe, generalized myasthenia gravis. Muscle Nerve. 2002. 26:31–36.
Article43. Drachman DB, Adams RN, Hu R, Jones RJ, Brodsky RA. Rebooting the immune system with high-dose cyclophosphamide for treatment of refractory myasthenia gravis. Ann N Y Acad Sci. 2008. 1132:305–314.
Article44. Bonifati DM, Angelini C. Long-term cyclosporine treatment in a group of severe myasthenia gravis patients. J Neurol. 1997. 244:542–547.
Article45. Lavrnic D, Vujic A, Rakocevic-Stojanovic V, Stevic Z, Basta I, Pavlovic S, et al. Cyclosporine in the treatment of myasthenia gravis. Acta Neurol Scand. 2005. 111:247–252.
Article46. Tindall RS, Rollins JA, Phillips JT, Greenlee RG, Wells L, Belendiuk G. Preliminary results of a double-blind, randomized, placebo-controlled trial of cyclosporine in myasthenia gravis. N Engl J Med. 1987. 316:719–724.
Article47. Tindall RS, Phillips JT, Rollins JA, Wells L, Hall K. A clinical therapeutic trial of cyclosporine in myasthenia gravis. Ann N Y Acad Sci. 1993. 681:539–551.
Article48. Jarosz JM, Howlett DC, Cox TC, Bingham JB. Cyclosporine-related reversible posterior leukoencephalopathy: MRI. Neuroradiology. 1997. 39:711–715.
Article49. Konishi T, Yoshiyama Y, Takamori M, Yagi K, Mukai E, Saida T, et al. Clinical study of FK506 in patients with myasthenia gravis. Muscle Nerve. 2003. 28:570–574.
Article50. Ponseti JM, Gamez J, Azem J, López-Cano M, Vilallonga R, Armengol M. Tacrolimus for myasthenia gravis: a clinical study of 212 patients. Ann N Y Acad Sci. 2008. 1132:254–263.51. Zebardast N, Patwa HS, Novella SP, Goldstein JM. Rituximab in the management of refractory myasthenia gravis. Muscle Nerve. 2010. 41:375–378.
Article52. Maddison P, McConville J, Farrugia ME, Davies N, Rose M, Norwood F, et al. The use of rituximab in myasthenia gravis and Lambert-Eaton myasthenic syndrome. J Neurol Neurosurg Psychiatry. 2011. 82:671–673.
Article53. Carson KR, Focosi D, Major EO, Petrini M, Richey EA, West DP, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 2009. 10:816–824.
Article54. Punga AR, Stålberg E. Acetylcholinesterase inhibitors in MG: to be or not to be? Muscle Nerve. 2009. 39:724–728.
Article55. Argov Z, McKee D, Agus S, Brawer S, Shlomowitz N, Yoseph OB, et al. Treatment of human myasthenia gravis with oral antisense suppression of acetylcholinesterase. Neurology. 2007. 69:699–700.
Article56. Rowin J, Meriggioli MN, Tüzün E, Leurgans S, Christadoss P. Etanercept treatment in corticosteroid-dependent myasthenia gravis. Neurology. 2004. 63:2390–2392.
Article57. Fee DB, Kasarskis EJ. Myasthenia gravis associated with etanercept therapy. Muscle Nerve. 2009. 39:866–870.
Article58. Walport MJ. Complement. First of two parts. N Engl J Med. 2001. 344:1058–1066.59. Soltys J, Kusner LL, Young A, Richmonds C, Hatala D, Gong B, et al. Novel complement inhibitor limits severity of experimentally myasthenia gravis. Ann Neurol. 2009. 65:67–75.
Article60. Strober J, Cowan MJ, Horn BN. Allogeneic hematopoietic cell transplantation for refractory myasthenia gravis. Arch Neurol. 2009. 66:659–661.
Article