J Clin Neurol.
2011 Jun;7(2):60-68. 10.3988/jcn.2011.7.2.60.
Biomarkers Predicting Alzheimer's Disease in Cognitively Normal Aging
- Affiliations
-
- 1Department of Neurology, Bucheon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Bucheon, Korea.
- 2Knight Alzheimer's Disease Research Center, Washington University School of Medicine, St. Louis, MO, USA. morrisj@abraxas.wustl.edu
- 3Department of Neurology, Washington University School of Medicine, St. Louis, MO, USA.
- KMID: 2178968
- DOI: http://doi.org/10.3988/jcn.2011.7.2.60
Abstract
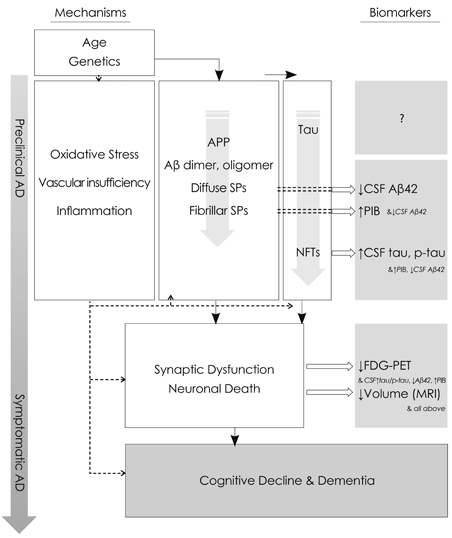
- The pathophysiologic process of Alzheimer's disease (AD) begins years before the diagnosis of clinical dementia. This concept of preclinical AD has arisen from the observation of AD pathologic findings such as senile plaques and neurofibrillary tangles in the brains of people who at the time of death had normal cognitive function. Recent advances in biomarker studies now provide the ability to detect the pathologic changes of AD, which are antecedent to symptoms of the illness, in cognitively normal individuals. Functional and structural brain alterations that begin with amyloid-beta accumulation already show the patterns of abnormality seen in individuals with dementia due to AD. The presence of preclinical AD provides a critical opportunity for potential interventions with disease-modifying therapy. This review focuses on the studies of antecedent biomarkers for preclinical AD.
Keyword
Figure
Cited by 1 articles
-
Morphological and Microstructural Changes of the Hippocampus in Early MCI: A Study Utilizing the Alzheimer's Disease Neuroimaging Initiative Database
Peter Lee, Hojin Ryoo, Jinah Park, Yong Jeong,
J Clin Neurol. 2017;13(2):144-154. doi: 10.3988/jcn.2017.13.2.144.
Reference
-
1. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991. 82:239–259.
Article2. Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch Neurol. 1985. 42:1097–1105.
Article3. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991. 41:479–486.
Article4. Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997. 56:1095–1097.
Article5. Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999. 45:358–368.
Article6. Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer's disease. EMBO Mol Med. 2009. 1:371–380.
Article7. Morris JC, McKeel DW Jr, Storandt M, Rubin EH, Price JL, Grant EA, et al. Very mild Alzheimer's disease: informant-based clinical, psychometric, and pathologic distinction from normal aging. Neurology. 1991. 41:469–478.
Article8. Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001. 58:1395–1402.
Article9. Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001. 17:101–118.
Article10. Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Medical Research Council Cognitive Function and Ageing Study. Age, neuropathology, and dementia. N Engl J Med. 2009. 360:2302–2309.
Article11. Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006. 20:112–117.
Article12. Mortimer JA, Borenstein AR, Gosche KM, Snowdon DA. Very early detection of Alzheimer neuropathology and the role of brain reserve in modifying its clinical expression. J Geriatr Psychiatry Neurol. 2005. 18:218–223.
Article13. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004. 256:183–194.
Article14. Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007. 6:734–746.
Article15. Craig-Schapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer's disease. Neurobiol Dis. 2009. 35:128–140.
Article16. Tarawneh R, Holtzman DM. Biomarkers in translational research of Alzheimer's disease. Neuropharmacology. 2010. 59:310–322.
Article17. Haass C, Selkoe DJ. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993. 75:1039–1042.
Article18. van Gool WA, Kuiper MA, Walstra GJ, Wolters EC, Bolhuis PA. Concentrations of amyloid beta protein in cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995. 37:277–279.
Article19. Shoji M, Matsubara E, Kanai M, Watanabe M, Nakamura T, Tomidokoro Y, et al. Combination assay of CSF tau, A beta 1-40 and A beta 1-42(43) as a biochemical marker of Alzheimer's disease. J Neurol Sci. 1998. 158:134–140.
Article20. Verbeek MM, Kremer BP, Rikkert MO, Van Domburg PH, Skehan ME, Greenberg SM. Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann Neurol. 2009. 66:245–249.
Article21. Blennow K, Vanmechelen E, Hampel H. CSF total tau, Abeta42 and phosphorylated tau protein as biomarkers for Alzheimer's disease. Mol Neurobiol. 2001. 24:87–97.22. Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, et al. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci USA. 1993. 90:10836–10840.
Article23. Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995. 38:643–648.
Article24. Riemenschneider M, Wagenpfeil S, Diehl J, Lautenschlager N, Theml T, Heldmann B, et al. Tau and Abeta42 protein in CSF of patients with frontotemporal degeneration. Neurology. 2002. 58:1622–1628.
Article25. Sjögren M, Minthon L, Davidsson P, Granérus A-K, Clarberg A, Vanderstichele H, et al. CSF levels of tau, beta-amyloid(1-42) and GAP-43 in frontotemporal dementia, other types of dementia and normal aging. J Neural Transm. 2000. 107:563–579.
Article26. Clark CM, Xie S, Chittams J, Ewbank D, Peskind E, Galasko D, et al. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol. 2003. 60:1696–1702.27. Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology. 2007. 68:666–669.
Article28. Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, et al. Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003. 289:2094–2103.29. Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007. 68:1718–1725.30. Kosaka T, Imagawa M, Seki K, Arai H, Sasaki H, Tsuji S, et al. The beta APP717 Alzheimer mutation increases the percentage of plasma amyloid-beta protein ending at A beta42(43). Neurology. 1997. 48:741–745.
Article31. Fukumoto H, Tennis M, Locascio JJ, Hyman BT, Growdon JH, Irizarry MC. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol. 2003. 60:958–964.
Article32. Mehta PD, Pirttilä T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000. 57:100–105.
Article33. Vanderstichele H, Van Kerschaver E, Hesse C, Davidsson P, Buyse MA, Andreasen N, et al. Standardization of measurement of beta-amyloid(1-42) in cerebrospinal fluid and plasma. Amyloid. 2000. 7:245–258.
Article34. Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007. 64:343–349.
Article35. Itoh N, Arai H, Urakami K, Ishiguro K, Ohno H, Hampel H, et al. Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer's disease. Ann Neurol. 2001. 50:150–156.
Article36. Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009. 65:403–413.
Article37. Mandelkow EM, Mandelkow E. Tau in Alzheimer's disease. Trends Cell Biol. 1998. 8:425–427.
Article38. Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003. 2:605–613.
Article39. Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004. 61:95–102.
Article40. Buerger K, Zinkowski R, Teipel SJ, Tapiola T, Arai H, Blennow K, et al. Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol. 2002. 59:1267–1272.
Article41. De Leon MJ, George AE, Golomb J, Tarshish C, Convit A, Kluger A, et al. Frequency of hippocampal formation atrophy in normal aging and Alzheimer's disease. Neurobiol Aging. 1997. 18:1–11.
Article42. Jobst KA, Smith AD, Barker CS, Wear A, King EM, Smith A, et al. Association of atrophy of the medial temporal lobe with reduced blood flow in the posterior parietotemporal cortex in patients with a clinical and pathological diagnosis of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1992. 55:190–194.
Article43. Bosscher L, Scheltens PH, editors. MRI of the temporal lobe. Evidence based dementia. 2001. Oxford: Blackwell.44. Tapiola T, Pennanen C, Tapiola M, Tervo S, Kivipelto M, Hänninen T, et al. MRI of hippocampus and entorhinal cortex in mild cognitive impairment: a follow-up study. Neurobiol Aging. 2008. 29:31–38.
Article45. deToledo-Morrell L, Stoub TR, Bulgakova M, Wilson RS, Bennett DA, Leurgans S, et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging. 2004. 25:1197–1203.
Article46. Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, et al. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003. 23:994–1005.
Article47. Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005. 64:1032–1039.
Article48. Carlson NE, Moore MM, Dame A, Howieson D, Silbert LC, Quinn JF, et al. Trajectories of brain loss in aging and the development of cognitive impairment. Neurology. 2008. 70:828–833.
Article49. Bobinski M, de Leon MJ, Wegiel J, Desanti S, Convit A, Saint Louis LA, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer's disease. Neuroscience. 2000. 95:721–725.
Article50. Zarow C, Vinters HV, Ellis WG, Weiner MW, Mungas D, White L, et al. Correlates of hippocampal neuron number in Alzheimer's disease and ischemic vascular dementia. Ann Neurol. 2005. 57:896–903.
Article51. Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009. 73:294–301.
Article52. Sluimer JD, Bouwman FH, Vrenken H, Blankenstein MA, Barkhof F, van der Flier WM, et al. Whole-brain atrophy rate and CSF biomarker levels in MCI and AD: a longitudinal study. Neurobiol Aging. 2010. 31:758–764.
Article53. Coleman RE. Positron emission tomography diagnosis of Alzheimer's disease. Neuroimaging Clin N Am. 2005. 15:837–846. x
Article54. Patwardhan MB, McCrory DC, Matchar DB, Samsa GP, Rutschmann OT. Alzheimer disease: operating characteristics of PET--a meta-analysis. Radiology. 2004. 231:73–80.
Article55. de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET). Proc Natl Acad Sci U S A. 2001. 98:10966–10971.
Article56. Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003. 30:1104–1113.
Article57. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993. 43:2412–2414.58. de Leon MJ, Mosconi L, Blennow K, DeSanti S, Zinkowski R, Mehta PD, et al. maging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann N Y Acad Sci. 2007. 1097:114–145.
Article59. Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009. 73:1193–1199.
Article60. Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003. 46:2740–2754.
Article61. Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004. 55:306–319.
Article62. Engler H, Forsberg A, Almkvist O, Blomquist G, Larsson E, Savitcheva I, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain. 2006. 129:2856–2866.
Article63. Jack CR Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008. 131:665–680.
Article64. Choi SR, Golding G, Zhuang Z, Zhang W, Lim N, Hefti F, et al. Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain. J Nucl Med. 2009. 50:1887–1894.
Article65. Sperling R, Johnson K, Pontecorvo M, Safirstein B, Farmer M, Holub R, et al. PET imaging of β-amyloid with florpiramine F18 (18F-AV-45): preliminary results from a phase II study of cognitive normal elderly subjects, individuals with mild cognitive impairment, and patients with a clinical diagnosis of Alzheimer's disease. Alzheimers Dement. 2009. 5:1997.66. Mintun M, Saha K, Fleisher A, Schneider J, Beach T, Bedell B, et al. Florbetapir (18F-AV-45) PET imaging of β-amyloid plaques is highly correlated with histopathological assays at autopsy. J Nucl Med. 2010. 51:387.67. Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006. 67:446–452.
Article68. Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006. 59:512–519.
Article69. Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009. 66:1469–1475.
Article70. Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, et al. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009. 65:176–183.
Article71. Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, et al. Brain atrophy in healthy aging is related to CSF levels of Aβ1-42. Cereb Cortex. 2010. 20:2069–2079.
Article72. Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007. 69:631–639.
Article73. Cairns NJ, Ikonomovic MD, Benzinger T, Storandt M, Fagan AM, Shah AR, et al. Absence of Pittsburgh compound B detection of cerebral amyloid beta in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease: a case report. Arch Neurol. 2009. 66:1557–1562.74. Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010. 67:122–131.
Article75. Moonis M, Swearer JM, Dayaw MP, St George-Hyslop P, Rogaeva E, Kawarai T, et al. Familial Alzheimer disease: decreases in CSF Abeta42 levels precede cognitive decline. Neurology. 2005. 65:323–325.
Article76. Ringman JM, Younkin SG, Pratico D, Seltzer W, Cole GM, Geschwind DH, et al. Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology. 2008. 71:85–92.
Article77. Godbolt AK, Cipolotti L, Watt H, Fox NC, Janssen JC, Rossor MN. The natural history of Alzheimer disease: a longitudinal presymptomatic and symptomatic study of a familial cohort. Arch Neurol. 2004. 61:1743–1748.78. Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004. 25:641–650.
Article79. Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002. 59:1737–1746.80. Silverman JM, Raiford K, Edland S, Fillenbaum G, Morris JC, Clark CM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part VI. Family history assessment: a multicenter study of first-degree relatives of Alzheimer's disease probands and nondemented spouse controls. Neurology. 1994. 44:1253–1259.
Article81. Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol. 2009. 66:1254–1259.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroimaging Markers for Alzheimer's Disease and Mild Cognitive Impairment in Alzheimer's Disease Neuroimaging Initiative (ADNI)
- Subjective Cognitive Complaints in Cognitively Normal Patients With Parkinson’s Disease: A Systematic Review
- Risk Factors for Subjective Memory Impairment in Cognitively Normal Elderly
- New Diagnostic Criteria for Alzheimer's Disease : NIA-AA Diagnostic Guidelines
- A New Biological Definition of Alzheimer's Disease: Introduction of 2018 National Institute on Aging-Alzheimer's Association Research Framework