Diabetes Metab J.
2015 Dec;39(6):489-497. 10.4093/dmj.2015.39.6.489.
Clinical Characteristics and Metabolic Predictors of Rapid Responders to Dipeptidyl Peptidase-4 Inhibitor as an Add-on Therapy to Sulfonylurea and Metformin
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. drshchoi@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea.
- 3Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea.
- 4Department of Internal Medicine, Konkuk University Chungju Hospital, Konkuk University School of Medicine, Chungju, Korea.
- 5Department of Internal Medicine, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea.
- 6Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- KMID: 2174001
- DOI: http://doi.org/10.4093/dmj.2015.39.6.489
Abstract
- BACKGROUND
Dipeptidyl peptidase-4 (DPP-4) inhibitor add-on therapy is a new option for patients with inadequately controlled type 2 diabetes who are taking combined metformin and sulfonylurea (SU). We evaluated the efficacy and safety of this triple therapy and the characteristics of rapid responders and hypoglycemia-prone patients.
METHODS
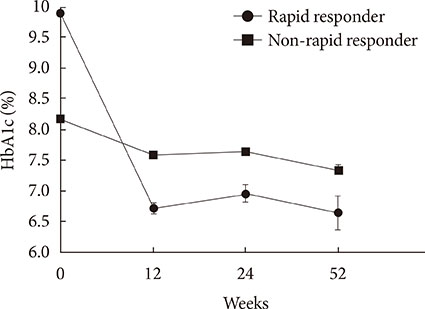
We included 807 patients with type 2 diabetes who were prescribed a newly added DPP-4 inhibitor to ongoing metformin and SU in 2009 to 2011. Glycemia and other metabolic parameters at baseline, 12, 24, and 52 weeks, as well as episodes of hypoglycemia were analyzed. Rapid responders were defined as patients with > or =25% reduction in glycosylated hemoglobin (HbA1c) within 12 weeks.
RESULTS
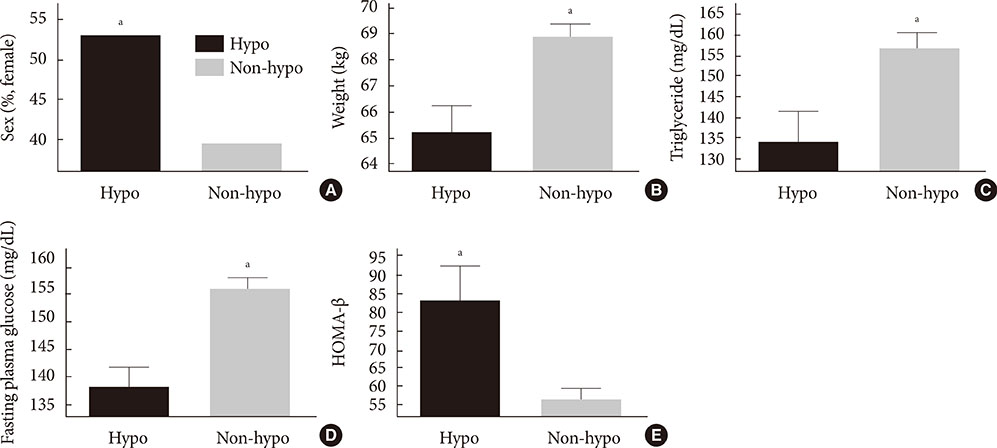
At baseline, while on the submaximal metformin and SU combination, the mean HbA1c level was 8.4%. Twelve weeks after initiation of DPP-4 inhibitor add-on, 269 patients (34.4%) achieved an HbA1c level < or =7%. Sixty-six patients (8.2%, 47 men) were rapid responders. The duration of diabetes was shorter in rapid responders, and their baseline fasting plasma glucose (FPG), HbA1c, C-peptide, and homeostasis model assessment of insulin resistance were significantly higher. Patients who experienced hypoglycemia after taking DPP-4 inhibitor add-on were more likely to be female, to have a lower body weight and lower triglyceride and FPG levels, and to have higher homeostasis model assessment of beta-cells.
CONCLUSION
An oral hypoglycemic triple agent combination including a DPP-4 inhibitor was effective in patients with uncontrolled diabetes. Proactive dose reduction of SU should be considered when a DPP-4 inhibitor is added for rapid responders and hypoglycemia-prone patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Waget A, Cabou C, Masseboeuf M, Cattan P, Armanet M, Karaca M, Castel J, Garret C, Payros G, Maida A, Sulpice T, Holst JJ, Drucker DJ, Magnan C, Burcelin R. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology. 2011; 152:3018–3029.2. Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007; 28:187–218.3. Turner RC, Cull CA, Frighi V, Holman RR. UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999; 281:2005–2012.4. Polonsky WH, Hajos TR, Dain MP, Snoek FJ. Are patients with type 2 diabetes reluctant to start insulin therapy? An examination of the scope and underpinnings of psychological insulin resistance in a large, international population. Curr Med Res Opin. 2011; 27:1169–1174.5. Harashima SI, Ogura M, Tanaka D, Fukushima T, Wang Y, Koizumi T, Aono M, Murata Y, Seike M, Inagaki N. Sitagliptin add-on to low dosage sulphonylureas: efficacy and safety of combination therapy on glycaemic control and insulin secretion capacity in type 2 diabetes. Int J Clin Pract. 2012; 66:465–476.6. Hirao K, Maeda H, Shirabe S, Yamamoto R, Hirao T, Hirao S, Yamauchi M, Arai K. Combination therapy with a dipeptidyl peptidase-4 inhibitor, sulfonylurea, and metformin markedly improves HbA1c levels in Japanese patients with type 2 diabetes mellitus. Jpn Clin Med. 2012; 3:1–7.7. Rathmann W, Kostev K, Gruenberger JB, Dworak M, Bader G, Giani G. Treatment persistence, hypoglycaemia and clinical outcomes in type 2 diabetes patients with dipeptidyl peptidase-4 inhibitors and sulphonylureas: a primary care database analysis. Diabetes Obes Metab. 2013; 15:55–61.8. Monami M, Cremasco F, Lamanna C, Marchionni N, Mannucci E. Predictors of response to dipeptidyl peptidase-4 inhibitors: evidence from randomized clinical trials. Diabetes Metab Res Rev. 2011; 27:362–372.9. Kim SA, Shim WH, Lee EH, Lee YM, Beom SH, Kim ES, Yoo JS, Nam JS, Cho MH, Park JS, Ahn CW, Kim KR. Predictive clinical parameters for the therapeutic efficacy of sitagliptin in Korean type 2 diabetes mellitus. Diabetes Metab J. 2011; 35:159–165.10. Kim WJ, Park CY, Jeong EH, Seo JY, Seol JS, Park SE, Rhee EJ, Lee WY, Oh KW, Park SW, Kim SW. Retrospective analysis on the efficacy, safety and treatment failure group of sitagliptin for mean 10-month duration. Diabetes Metab J. 2011; 35:290–297.11. Kanazu S, Horie Y, Narukawa M, Nonaka K, Taniguchi T, Arjona Ferreira JC, Takeuchi M. Predicting steady-state HbA1c responses to sitagliptin in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2009; 11:813–818.12. Chung HS, Lee MK. Efficacy of sitagliptin when added to ongoing therapy in Korean subjects with type 2 diabetes mellitus. Diabetes Metab J. 2011; 35:411–417.13. Hamaguchi T, Koga M, Murai J, Saito H, Tamada D, Kurebayashi S, Katsuno T, Miyagawa J, Namba M. Estimation of HbA1c response to sitagliptin by change in glycated albumin level for 2 weeks. J Diabetes Investig. 2012; 3:175–178.14. Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007; 9:733–745.15. Sato D, Sato Y, Masuda S, Kimura H. Impact of the sitagliptin alert on prescription of oral antihyperglycemic drugs in Japan. Int J Clin Pharm. 2012; 34:917–924.16. Kimura T, Shiosakai K, Takeda Y, Takahashi S, Kobayashi M, Sakaguchi M. Quantitative evaluation of compliance with recommendation for sulfonylurea dose co-administered with DPP-4 inhibitors in Japan. Pharmaceutics. 2012; 4:479–493.17. Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007; 30:1979–1987.18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419.19. Nomiyama T, Akehi Y, Takenoshita H, Nagaishi R, Terawaki Y, Nagasako H, Kudo T, Kodera T, Kobayashi K, Urata H, Yanase T. CHAT. Contributing factors related to efficacy of the dipeptidyl peptidase-4 inhibitor sitagliptin in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2012; 95:e27–e28.20. Seino S, Zhang CL, Shibasaki T. Sulfonylurea action re-revisited. J Diabetes Investig. 2010; 1:37–39.21. Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998; 282:2275–2279.22. de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998; 396:474–477.23. Ishii H, Sato Y, Takei M, Nishio S, Komatsu M. Glucose-incretin interaction revisited. Endocr J. 2011; 58:519–525.24. Hong ES, Khang AR, Yoon JW, Kang SM, Choi SH, Park KS, Jang HC, Shin H, Walford GA, Lim S. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012; 14:795–802.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiovascular Outcomes Comparison of Dipeptidyl Peptidase-4 Inhibitors versus Sulfonylurea as Add-on Therapy for Type 2 Diabetes Mellitus: a Meta-Analysis
- Efficacy of Body Weight Reduction on the SGLT2 Inhibitor in People with Type 2 Diabetes Mellitus
- Dipeptidyl Peptidase-4 Inhibitors versus Other Antidiabetic Drugs Added to Metformin Monotherapy in Diabetic Retinopathy Progression: A Real World-Based Cohort Study
- Sodium Glucose Cotransporter-2 Inhibitors as an Add-on Therapy to Metformin Plus Dipeptidyl Peptidase-4 Inhibitor in Patients with Type 2 Diabetes
- Correction to: “Cardiovascular Outcomes Comparison of Dipeptidyl Peptidase-4 Inhibitors Versus Sulfonylurea as Add-on Therapy for Type 2 Diabetes Mellitus: A Meta-Analysis”