Chonnam Med J.
2011 Dec;47(3):150-154. 10.4068/cmj.2011.47.3.150.
Clinical Progress of Epilepsy in Children with Tuberous Sclerosis: Prognostic Factors for Seizure Outcome
- Affiliations
-
- 1Department of Pediatrics, Chonnam National University Medical School, Gwangju, Korea. yjwoo@jnu.ac.kr
- KMID: 2172223
- DOI: http://doi.org/10.4068/cmj.2011.47.3.150
Abstract
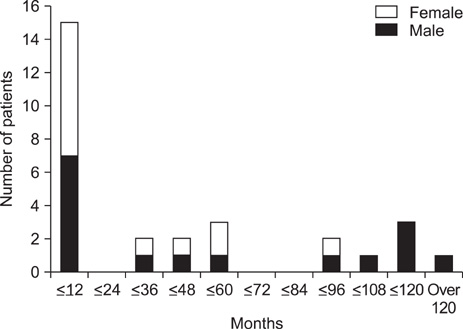
- The incidence and outcome of epilepsy in tuberous sclerosis (TS) patients have not yet been thoroughly investigated. The aim of this study was to evaluate the clinical features and prognosis of epileptic seizures associated with TS. The medical records of 29 patients who satisfied the diagnostic criteria for TS and were followed up for at least 2 years at the Department of Pediatrics, Chonnam National University Hospital (CNUH), between January 2000 and December 2010 were reviewed. Onset age of seizure, initial electroencephalography (EEG) findings, and efficacy of treatment were evaluated. Brain imaging studies were reanalyzed to determine the number of cortical tubers and subependymal nodules present. A total of 26 (89.6%) cases presented with seizures. In the seizure-controlled group (n=9, 34.6%), the mean number of cortical tubers was 4.5 (range, 0-16) and the mean number of subependymal nodules was 6.2 (range, 0-14). Initial EEG identified epileptiform discharges in 4 (44.5%) of these cases. In the seizure-sustained group (n=17, 58.6%), 10 patients had initial seizures before 1 year of age. In this group, the mean number of cortical tubers was 6.0 (range, 0-20) and the mean number of subependymal nodules was 6.0 (range, 1-11). A total of 15 (88.2%) had epileptiform discharges on their initial EEGs. In three patients who did not show any seizures during the observation period, the mean number of cortical tubers was 1.3 (range, 0-2), and the mean number of subependymal nodules was 4.6 (range, 0-13). Medical intractability of epilepsy in conjunction with TS did not correlate with age at onset of seizure, the number of cortical tubers, or subependymal nodules, but was associated with initial EEG findings.
Keyword
MeSH Terms
Figure
Reference
-
1. Devlin LA, Shepherd CH, Crawford H, Morrison PJ. Tuberous sclerosis complex: clinical features, diagnosis, and prevalence within Northern Ireland. Dev Med Child Neurol. 2006. 48:495–499.
Article2. Piao C, Yu A, Li K, Wang Y, Qin W, Xue S. Cerebral diffusion tensor imaging in tuberous sclerosis. Eur J Radiol. 2009. 71:249–252.
Article3. Hwang YS, Yoon YS. Clinical study of tuberous sclerosis - focused on neurologic manifestation and cardiac echo findings. J Korean Pediatr Soc. 1991. 34:992–998.4. Staley BA, Vail EA, Thiele EA. Tuberous sclerosis complex: diagnostic challenges, presenting symptoms, and commonly missed signs. Pediatrics. 2011. 127:e117–e125.
Article5. Cho HR, Song SM, Yum MS, Lee EH, Jeong MH, Yoo HW, et al. Analysis of Overall Clinical Manifestations of Tuberous Sclerosis Complex Patients in the Childhood: Updated Diagnositic Criteria. J Korean Child Neurol Soc. 2009. 17:174–184.6. Kim KJ, Hwang YS. Features and outcomes of epileptic seizure in tuberous sclerosis. J Korean Child Neurol Soc. 1995. 2:93–96.7. Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998. 13:624–628.
Article8. Pampiglione G, Moynahan EJ. The tuberous sclerosis syndrome: clinical and EEG studies in 100 children. J Neurol Neurosurg Psychiatry. 1976. 39:666–673.
Article9. Husain AM, Foley CM, Legido A, Chandler DA, Miles DK, Grover WD. West syndrome in tuberous sclerosis complex. Pediatr Neurol. 2000. 23:233–235.
Article10. Baron Y, Barkovich AJ. MR imaging of tuberous sclerosis in neonates and young infants. AJNR Am J Neuroradiol. 1999. 20:907–916.11. Gallagher A, Madan N, Stemmer-Rachamimov A, Thiele EA. Progressive calcified tuber in a young male with tuberous sclerosis complex. Dev Med Child Neurol. 2010. 52:1062–1065.
Article12. Evans JC, Curtis J. The radiological appearances of tuberous sclerosis. Br J Radiol. 2000. 73:91–98.
Article13. Firat AK, Karakaş HM, Erdem G, Yakinci C, Biçak U. Diffusion weighted MR findings of brain involvement in tuberous sclerosis. Diagn Interv Radiol. 2006. 12:57–60.14. Chou IJ, Lin KL, Wong AM, Wang HS, Chou ML, Hung PC, et al. Neuroimaging correlation with neurological severity in tuberous sclerosis complex. Eur J Paediatr Neurol. 2008. 12:108–112.
Article15. Arulrajah S, Ertan G, Jordan L, Tekes A, Khaykin E, Izbudak I, et al. Magnetic resonance imaging and diffusion-weighted imaging of normal-appearing white matter in children and young adults with tuberous sclerosis complex. Neuroradiology. 2009. 51:781–786.
Article16. Major P, Rakowski S, Simon MV, Cheng ML, Eskandar E, Baron J, et al. Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia. 2009. 50:147–154.
Article17. Chugani DC, Chugani HT, Muzik O, Shah JR, Shah AK, Canady A, et al. Imaging epileptogenic tubers in children with tuberous sclerosis complex using alpha-[11C]methyl-L-tryptophan positron emission tomography. Ann Neurol. 1998. 44:858–866.
Article18. Curatolo P, Verdecchia M, Bombardieri R. Tuberous sclerosis complex: a review of neurological aspects. Eur J Paediatr Neurol. 2002. 6:15–23.
Article