Ann Dermatol.
2014 Apr;26(2):209-213. 10.5021/ad.2014.26.2.209.
Effect of Adenosine on Melanogenesis in B16 Cells and Zebrafish
- Affiliations
-
- 1Department of Dermatology, Research Institute of Medical Sciences, Chungnam National University School of Medicine, Daejeon, Korea. joon@cnu.ac.kr
- KMID: 2171657
- DOI: http://doi.org/10.5021/ad.2014.26.2.209
Abstract
- BACKGROUND
Adenosine is a nucleoside, in which an adenine molecule is attached to a ribofuranose sugar moiety. It can be released into the microenvironment by metabolically active cells, and then fulfills a multitude of functions in regulation of cell proliferation, by activating four subtypes of G protein-coupled adenosine receptors.
OBJECTIVE
In this study, we investigated the effect of adenosine on melanogenesis, using B16 melanoma cells.
METHODS
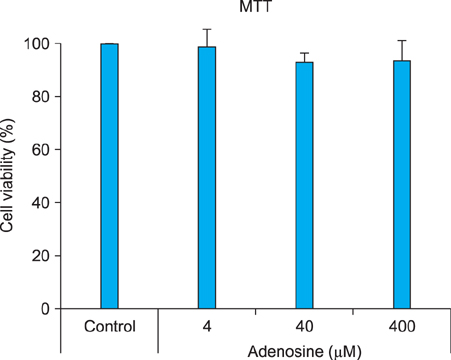
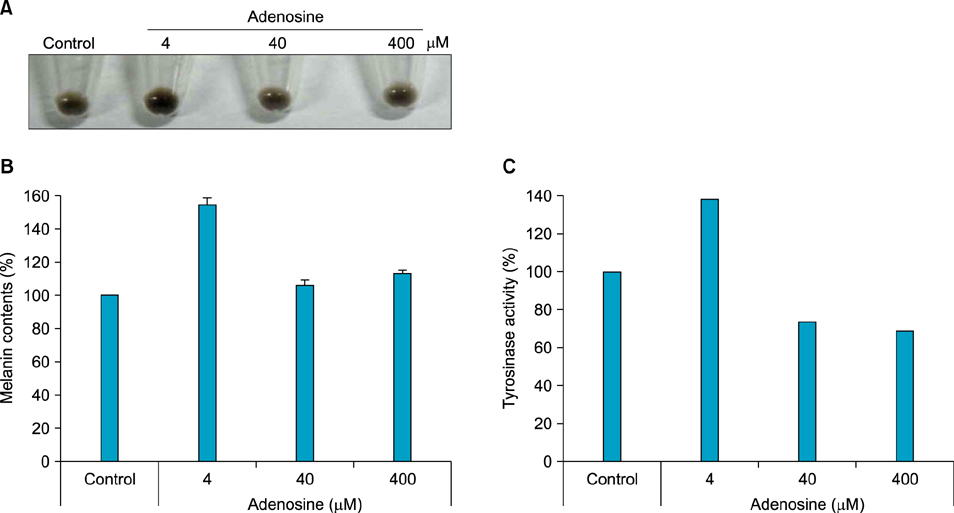
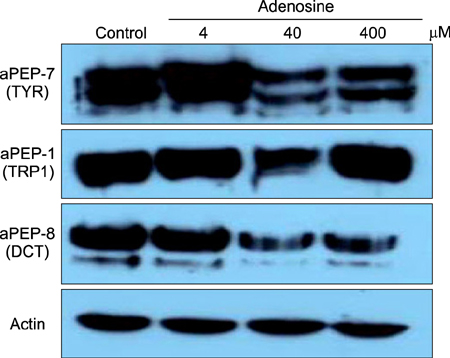
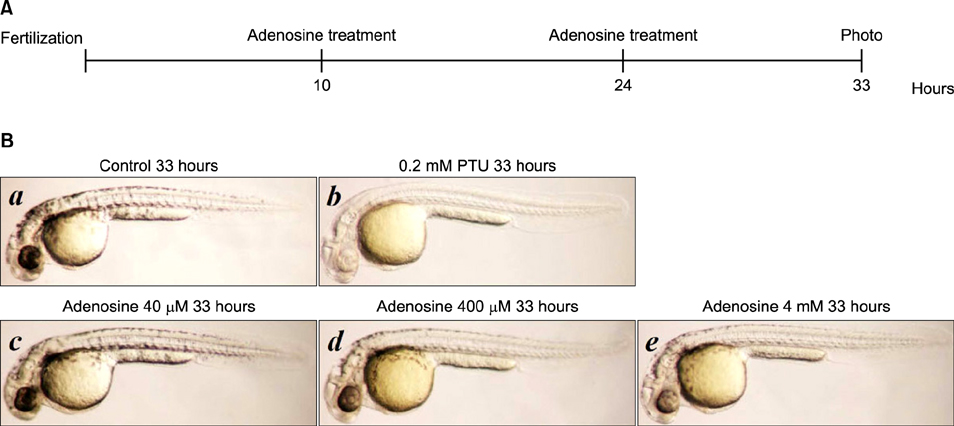
The toxic effects of adenosine on B16 melanoma cells were assessed. To understand the mechanism of the effect of adenosine on melanogenesis in B16 cells, melanin content and tyrosinase activity were measured. Tyrosinase, tyrosinase-related protein-1, and dopachrome tautomerase were monitored by Western blotting. Finally, adenosine was applied to zebrafish embryos, and its in vivo effect on pigmentation investigated.
RESULTS
At a low concentration, adenosine increased melanin content and tyrosinase activity, while a high dose of adenosine resulted in inhibition of tyrosinase activity. Western blotting showed that adenosine increased tyrosinase protein levels slightly, while high-dose adenosine decreased the expression of tyrosinase. In zebrafish tests, adenosine slightly inhibited body pigmentation.
CONCLUSION
In this study, we investigated the effect of adenosine on melanogenesis, using the well-established B16 melanoma cell and zebrafish models. The results suggest that adenosine may inhibit pigmentation, through negative regulation of tyrosinase.
Keyword
MeSH Terms
Figure
Reference
-
1. Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007; 21:976–994.
Article2. Yoon TJ, Lei TC, Yamaguchi Y, Batzer J, Wolber R, Hearing VJ. Reconstituted 3-dimensional human skin of various ethnic origins as an in vitro model for studies of pigmentation. Anal Biochem. 2003; 318:260–269.
Article3. Land EJ, Ramsden CA, Riley PA. Quinone chemistry and melanogenesis. Methods Enzymol. 2004; 378:88–109.
Article4. Wang N, Hebert DN. Tyrosinase maturation through the mammalian secretory pathway: bringing color to life. Pigment Cell Res. 2006; 19:3–18.
Article5. Buscà R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000; 13:60–69.
Article6. Wan P, Hu Y, He L. Regulation of melan < yte pivotal transcription factor MITF by some other transcription factors. Mol Cell Biochem. 2011; 354:241–246.
Article7. Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008; 7:759–770.
Article8. Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther. 2008; 117:123–140.
Article9. Ramakers BP, Riksen NP, van der, Smits P, Pickkers P. Modulation of innate immunity by adenosine receptor stimulation. Shock. 2011; 36:208–215.
Article10. Johnston-Cox HA, Koupenova M, Ravid K. A2 adenosine receptors and vascular pathologies. Arterioscler Thromb Vasc Biol. 2012; 32:870–878.
Article11. Streitová D, Hofer M, Holá J, Vacek A, Pospísil M. Adenosine A(1), A(2a), A(2b), and A(3) receptors in hematopoiesis.2. Expression of receptor mRNA in resting and lipopolysaccharide-activated mouse RAW 264.7 macrophages. Physiol Res. 2010; 59:139–144.
Article12. Choi TY, Kim JH, Ko DH, Kim CH, Hwang JS, Ahn S, et al. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007; 20:120–127.
Article13. Madi L, Rosenberg-Haggen B, Nyska A, Korenstein R. Enhancing pigmentation via activation of A3 adenosine receptors in B16 melanoma cells and in human skin explants. Exp Dermatol. 2013; 22:74–77.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stimulation of melanogenesis by glycyrrhizin in B16 melanoma cells
- Effect of Hypoxia on the Melanogenesis of Murine B16 Melanoma Cells
- Sphingosine 1-Phosphate Triggers Apoptotic Signal for B16 Melanoma Cells via ERK and Caspase Activation
- Downregulation of NFAT2 promotes melanogenesis in B16 melanoma cells
- Zebrafish and Mycobacterial infection