Anat Cell Biol.
2010 Dec;43(4):303-309. 10.5115/acb.2010.43.4.303.
Downregulation of NFAT2 promotes melanogenesis in B16 melanoma cells
- Affiliations
-
- 1Department of Anatomy, School of Medicine, Chungnam National University, Daejeon, Korea. yhlee@cnu.ac.kr
- 2Department of Dermatology, School of Medicine, Chungnam National University, Daejeon, Korea.
- 3Department of Physiology, School of Medicine, Chungnam National University, Daejeon, Korea.
- KMID: 1455344
- DOI: http://doi.org/10.5115/acb.2010.43.4.303
Abstract
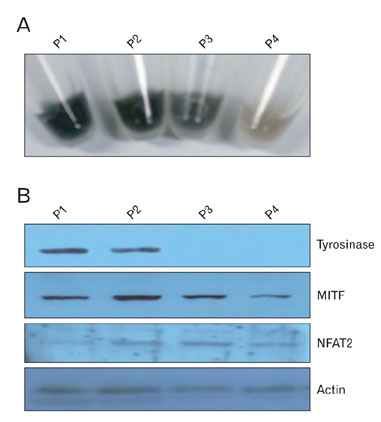
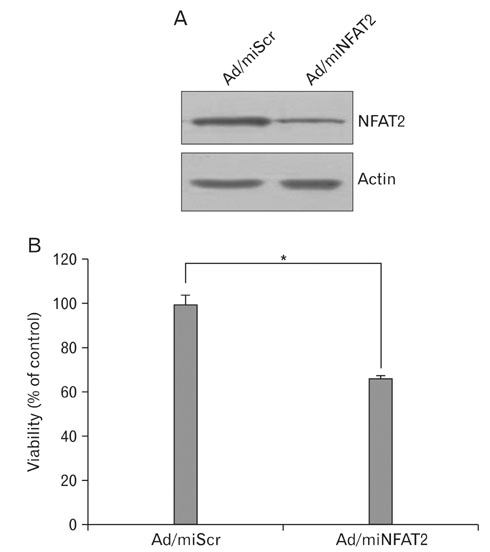
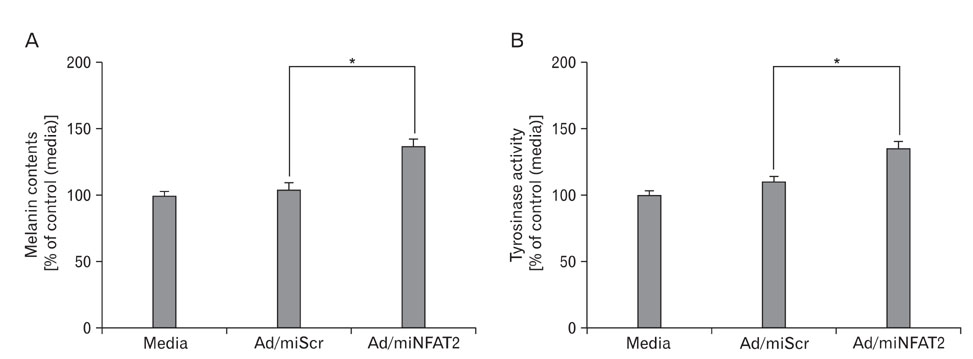
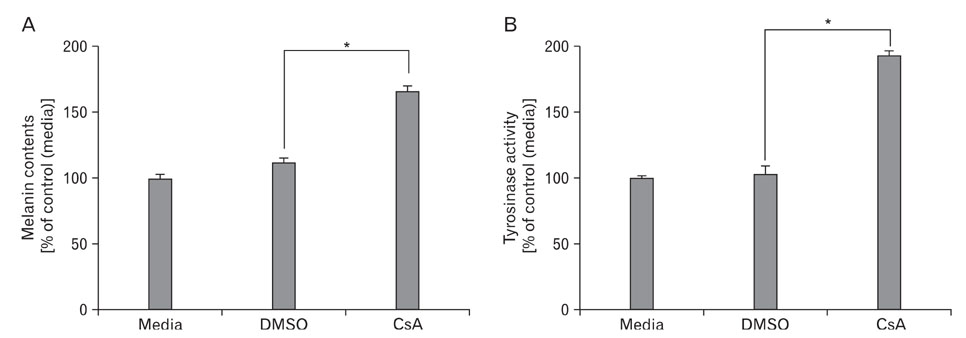
- Nuclear factor of activated T-cells (NFAT) proteins are, calcium-regulated transcription factors, key regulator of stimulation-dependent gene activation. In our microarray analysis for the genes expressed in human black and white hairs, NFAT2 was significantly upregulated in the white hair, compared to the black hair. The aim of this study was to investigate functional role of NFAT2 in melanogenesis. Western blot analysis was performed to investigate the expression of NFAT2 protein in B16 melanoma cells. Our data showed that NFAT2 expression was increased in the hypopigmented B16 cells, while tyrosinase and MITF expression was decreased. To investigate the potential role of NFAT2, the recombinant adenovirus expressing microRNA specific for NFAT2 was transduced into the cultured B16 melanoma cells. Consistently, inhibition of NFAT2 enhanced tyrosinase activity and melanin content. Moreover, cyclosporine A, which is known as a calcineurin inhibitor blocking NFAT activation, enhanced tyrosinase activity and melanin content. These data suggest that NFAT2 may play an important role in regulation of melanogenesis in melanocyte.
Keyword
MeSH Terms
-
Adenoviridae
Blotting, Western
Calcineurin
Cyclosporine
Down-Regulation
European Continental Ancestry Group
Hair
Humans
Hydroquinones
Melanins
Melanocytes
Melanoma, Experimental
Microarray Analysis
MicroRNAs
Monophenol Monooxygenase
NFATC Transcription Factors
Proteins
T-Lymphocytes
Transcription Factors
Transcriptional Activation
Calcineurin
Cyclosporine
Hydroquinones
Melanins
MicroRNAs
Monophenol Monooxygenase
NFATC Transcription Factors
Proteins
Transcription Factors
Figure
Reference
-
1. Al-Daraji WI, Afolayan J, Zelger BG, Abdellaoui A, Zelger B. Modulation of NFAT-5, an outlying member of the NFAT family, in human keratinocytes and skin. Am J Transl Res. 2009. 1:184–202.2. Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007. 21:976–994.3. Flockhart RJ, Armstrong JL, Reynolds NJ, Lovat PE. NFAT signalling is a novel target of oncogenic BRAF in metastatic melanoma. Br J Cancer. 2009. 101:1448–1455.4. Flockhart RJ, Diffey BL, Farr PM, Lloyd J, Reynolds NJ. NFAT regulates induction of COX-2 and apoptosis of keratinocytes in response to ultraviolet radiation exposure. FASEB J. 2008. 22:4218–4227.5. Friédmann PS, Wren FE, Matthews JN. Ultraviolet stimulated melanogenesis by human melanocytes is augmented by di-acyl glycerol but not TPA. J Cell Physiol. 1990. 142:334–341.6. Fung-Leung WP, Pope BL, Chourmouzis E, Panakos JA, Lau CY. Tepoxalin, a novel immunomodulatory compound, synergizes with CsA in suppression of graft-versus-host reaction and allogeneic skin graft rejection. Transplantation. 1995. 60:362–368.7. Gafter-Gvili A, Sredni B, Gal R, Gafter U, Kalechman Y. Cyclosporin A-induced hair growth in mice is associated with inhibition of calcineurin-dependent activation of NFAT in follicular keratinocytes. Am J Physiol Cell Physiol. 2003. 284:C1593–C1603.8. Gauthier M, Degnan BM. The transcription factor NF-kappaB in the demosponge Amphimedon queenslandica: insights on the evolutionary origin of the Rel homology domain. Dev Genes Evol. 2008. 218:23–32.9. Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008. 132:299–310.10. Hunt G, Todd C, Cresswell JE, Thody AJ. Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci. 1994. 107:205–211.11. Kadekaro AL, Wakamatsu K, Ito S, Abdel-Malek ZA. Cutaneous photoprotection and melanoma susceptibility: reaching beyond melanin content to the frontiers of DNA repair. Front Biosci. 2006. 11:2157–2173.12. Kuo CT, Leiden JM. Transcriptional regulation of T lymphocyte development and function. Annu Rev Immunol. 1999. 17:149–187.13. Macián F, López-Rodríguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001. 20:2476–2489.14. Maeda K, Yokokawa Y, Hatao M, Naganuma M, Tomita Y. Comparison of the melanogenesis in human black and light brown melanocytes. J Dermatol Sci. 1997. 14:199–206.15. Mammucari C, Tommasi di Vignano A, Sharov AA, et al. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell. 2005. 8:665–676.16. Murakami M, Matsuzaki F, Funaba M. Regulation of melanin synthesis by the TGF-beta family in B16 melanoma cells. Mol Biol Rep. 2009. 36:1247–1250.17. Na IK, Markley JC, Tsai JJ, et al. Concurrent visualization of trafficking, expansion, and activation of T lymphocytes and T-cell precursors in vivo. Blood. 2010. 116:e18–e25.18. Oh-hora M. Calcium signaling in the development and function of T-lineage cells. Immunol Rev. 2009. 231:210–224.19. Ouyang W, Hu Y, Li J, et al. Direct evidence for the critical role of NFAT3 in benzo[a]pyrene diol-epoxide-induced cell transformation through mediation of inflammatory cytokine TNF induction in mouse epidermal Cl41 cells. Carcinogenesis. 2007. 28:2218–2226.20. Pores-Fernando AT, Zweifach A. Calcium influx and signaling in cytotoxic T-lymphocyte lytic granule exocytosis. Immunol Rev. 2009. 231:160–173.21. Rinne A, Banach K, Blatter LA. Regulation of nuclear factor of activated T cells (NFAT) in vascular endothelial cells. J Mol Cell Cardiol. 2009. 47:400–410.22. Roméro-Graillet C, Aberdam E, Biagoli N, Massabni W, Ortonne JP, Ballotti R. Ultraviolet B radiation acts through the nitric oxide and cGMP signal transduction pathway to stimulate melanogenesis in human melanocytes. J Biol Chem. 1996. 271:28052–28056.23. Santini MP, Talora C, Seki T, Bolgan L, Dotto GP. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation. Proc Natl Acad Sci U S A. 2001. 98:9575–9580.24. Sawada M, Terada N, Taniguchi H, Tateishi R, Mori Y. Cyclosporin A stimulates hair growth in nude mice. Lab Invest. 1987. 56:684–686.25. Shibahara S, Yasumoto K, Amae S, et al. Regulation of pigment cell-specific gene expression by MITF. Pigment Cell Res. 2000. 13:Suppl 8. 98–102.26. Wolfe SA, Zhou P, Dötsch V, et al. Unusual Rel-like architecture in the DNA-binding domain of the transcription factor NFATc. Nature. 1997. 385:172–176.27. Wu X, Nguyen BC, Dziunycz P, et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010. 465:368–372.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Adenosine on Melanogenesis in B16 Cells and Zebrafish
- Sphingosine 1-Phosphate Triggers Apoptotic Signal for B16 Melanoma Cells via ERK and Caspase Activation
- Stimulation of melanogenesis by glycyrrhizin in B16 melanoma cells
- Effect of Hypoxia on the Melanogenesis of Murine B16 Melanoma Cells
- Activation of transforming growth factor-β and epithelialmesenchymal transition enhance regulatory T cells-mediated metastasis