Ann Dermatol.
2015 Aug;27(4):446-449. 10.5021/ad.2015.27.4.446.
Erythrodermic Psoriasis Treated with Golimumab: A Case Report
- Affiliations
-
- 1Department of Dermatology, Pusan National University Hospital, Busan, Korea. dockbs@pusan.ac.kr
- 2Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- 3Department of Dermatology, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 4Biomedical Research Institute, Pusan National University Yangsan Hospital, Yangsan, Korea.
- KMID: 2171505
- DOI: http://doi.org/10.5021/ad.2015.27.4.446
Abstract
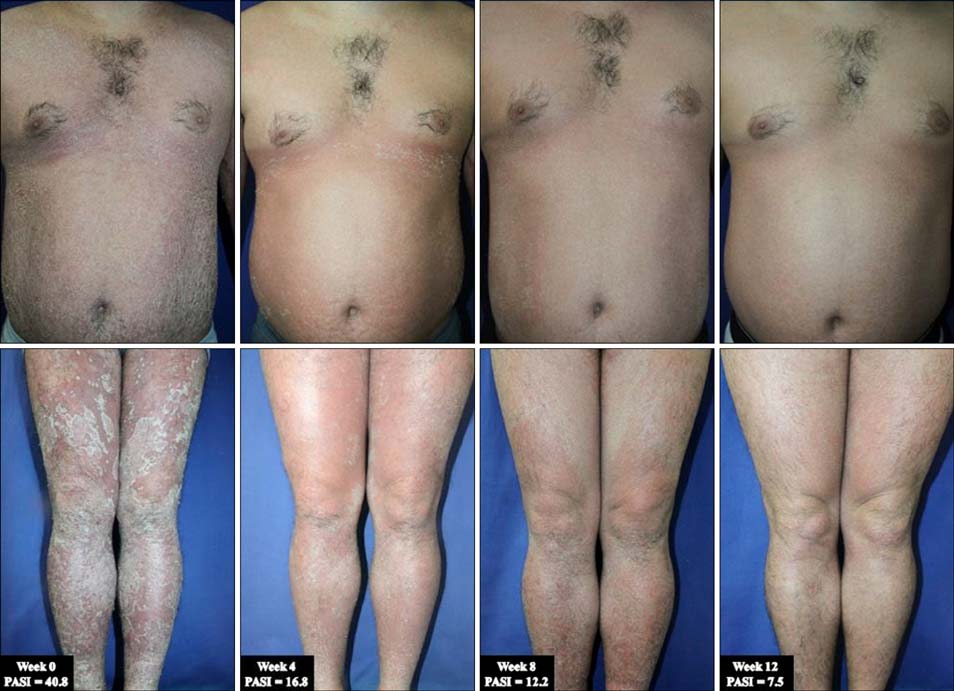
- Erythrodermic psoriasis (EP) is a very severe variant of psoriasis whose management poses a challenge to physicians, as currently available therapies often provide unsatisfactory results. Many biologics have been used to treat chronic plaque psoriasis, the most common form of psoriasis; however, their effectiveness for EP is poorly understood. A recently developed biologic, golimumab, has been extensively studied for the treatment of moderate-to-severe active rheumatoid arthritis, psoriatic arthritis, active ankylosing spondylitis, and chronic plaque psoriasis. However, no clinical trials have been performed for EP. Here, we report the case of a 32-year-old man who presented with severe psoriasis that previously failed to respond satisfactorily to methotrexate, cyclosporine, retinoid, narrow-band ultraviolet B phototherapy, and topical agents (i.e., steroids and calcipotriol). Skin lesions worsened progressively and developed into erythroderma. Psoriatic arthritis was also detected. Conventional therapies lacked efficacy. Therefore, we administered golimumab 50 mg. The skin lesions improved significantly according to the Psoriasis Area and Severity Index score after the first administration; lesions improved further throughout the treatment course. Although additional studies are required to fully evaluate the efficacy and safety of golimumab, this agent may be an alternative treatment strategy for some patients with recalcitrant EP.
MeSH Terms
Figure
Reference
-
1. Esposito M, Mazzotta A, de Felice C, Papoutsaki M, Chimenti S. Treatment of erythrodermic psoriasis with etanercept. Br J Dermatol. 2006; 155:156–159.
Article2. Boyce EG, Halilovic J, Stan-Ugbene O. Golimumab: review of the efficacy and tolerability of a recently approved tumor necrosis factor-α inhibitor. Clin Ther. 2010; 32:1681–1703.
Article3. Boyd AS, Menter A. Erythrodermic psoriasis. Precipitating factors, course, and prognosis in 50 patients. J Am Acad Dermatol. 1989; 21:985–991.4. Jalal O, Houass S, Laissaoui K, Hocar O, Charioui S, Amal S. Severe psoriasis: 160 cases. Ann Dermatol Venereol. 2005; 132:126–128.5. Marks J. Erythrodermas and uric acid aberrations in psoriasis. In : Farber EM, Cox AJ, editors. Psoriasis: proceedings of the International Symposium Stanford University. Stanford, CA: Stanford University Press;1971. p. 89–98.6. Heikkilä H, Ranki A, Cajanus S, Karvonen SL. Infliximab combined with methotrexate as long-term treatment for erythrodermic psoriasis. Arch Dermatol. 2005; 141:1607–1610.
Article7. Poulalhon N, Begon E, Lebbé C, Lioté F, Lahfa M, Bengoufa D, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007; 156:329–336.
Article8. Takahashi MD, Castro LG, Romiti R. Infliximab, as sole or combined therapy, induces rapid clearing of erythrodermic psoriasis. Br J Dermatol. 2007; 157:828–831.
Article9. Richetta AG, Maiani E, Carlomagno V, Carboni V, Mattozzi C, Giancristoforo S, et al. Treatment of erythrodermic psoriasis in HCV+ patient with adalimumab. Dermatol Ther. 2009; 22:Suppl 1. S16–S18.
Article10. Santos-Juanes J, Coto-Segura P, Mas-Vidal A, Galache Osuna C. Ustekinumab induces rapid clearing of erythrodermic psoriasis after failure of antitumour necrosis factor therapies. Br J Dermatol. 2010; 162:1144–1146.
Article11. Wang TS, Tsai TF. Clinical experience of ustekinumab in the treatment of erythrodermic psoriasis: a case series. J Dermatol. 2011; 38:1096–1099.
Article12. Viguier M, Pagès C, Aubin F, Delaporte E, Descamps V, Lok C, et al. Groupe Français de Recherche sur le Psoriasis. Efficacy and safety of biologics in erythrodermic psoriasis: a multicentre, retrospective study. Br J Dermatol. 2012; 167:417–423.
Article13. Lee EJ, Shin MK, Kim NI. A clinical trial of combination therapy with etanercept and low dose cyclosporine for the treatment of refractory psoriasis. Ann Dermatol. 2010; 22:138–142.
Article14. Lizzul PF, Aphale A, Malaviya R, Sun Y, Masud S, Dombrovskiy V, et al. Differential expression of phosphorylated NF-kappaB/RelA in normal and psoriatic epidermis and downregulation of NF-kappaB in response to treatment with etanercept. J Invest Dermatol. 2005; 124:1275–1283.
Article15. Kavanaugh A, Mease P. Treatment of psoriatic arthritis with tumor necrosis factor inhibitors: longer-term outcomes including enthesitis and dactylitis with golimumab treatment in the Longterm Extension of a Randomized, Placebo-controlled Study (GO-REVEAL). J Rheumatol Suppl. 2012; 89:90–93.
Article16. Zhou H, Jang H, Fleischmann RM, Bouman-Thio E, Xu Z, Marini JC, et al. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-alpha monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol. 2007; 47:383–396.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Erythrodermic Psoriasis with Etretinate
- Erythrodermic Psoriasis Improved by Ustekinumab: A Report of Two Cases
- A Case of Nail Psoriasis Improved by Treatment with Golimumab in a Psoriatic Arthritis Patient
- A Case of Psoriasiform Eruption Triggered by Tumor Necrosis Factor-alpha Antagonist Therapy
- A Case of Erythrodermic Form of Mycosis Fungoides