Endocrinol Metab.
2014 Jun;29(2):169-178. 10.3803/EnM.2014.29.2.169.
Highly Palatable Food during Adolescence Improves Anxiety-Like Behaviors and Hypothalamic-Pituitary-Adrenal Axis Dysfunction in Rats that Experienced Neonatal Maternal Separation
- Affiliations
-
- 1Program in Craniofacial Structure and Functional Biology, Department of Dental Science, Graduate School, Seoul National University School of Dentistry, Seoul, Korea.
- 2Department of Oral and Maxillofacial Surgery, Seoul National University School of Dentistry, Seoul, Korea.
- 3Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. jwjahng@snu.ac.kr
- KMID: 2169424
- DOI: http://doi.org/10.3803/EnM.2014.29.2.169
Abstract
- BACKGROUND
This study was conducted to examine the effects of ad libitum consumption of highly palatable food (HPF) during adolescence on the adverse behavioral outcome of neonatal maternal separation.
METHODS
Male Sprague-Dawley pups were separated from dam for 3 hours daily during the first 2 weeks of birth (maternal separation, MS) or left undisturbed (nonhandled, NH). Half of MS pups received free access to chocolate cookies in addition to ad libitum chow from postnatal day 28 (MS+HPF). Pups were subjected to behavioral tests during young adulthood. The plasma corticosterone response to stress challenge was analyzed by radioimmunoassay.
RESULTS
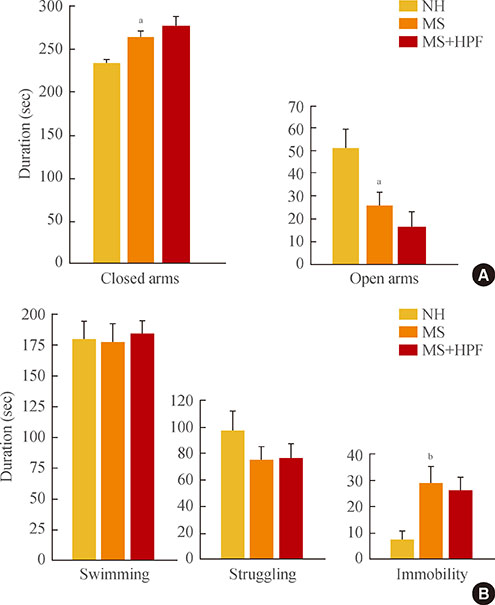
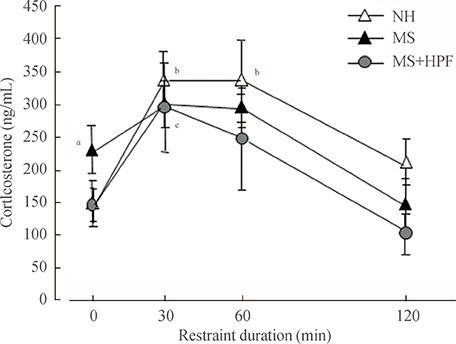
Daily caloric intake and body weight gain did not differ among the experimental groups. Ambulatory activities were decreased defecation activity and rostral grooming were increased in MS controls (fed with chow only) compared with NH rats. MS controls spent less time in open arms, and more time in closed arms during the elevated plus maze test, than NH rats. Immobility duration during the forced swim test was increased in MS controls compared with NH rats. Cookie access normalized the behavioral scores of ambulatory and defecation activities and grooming, but not the scores during the elevated plus maze and swim tests in MS rats. Stress-induced corticosterone increase was blunted in MS rats fed with chow only, and cookie access normalized it.
CONCLUSION
Prolonged access to HPF during adolescence and youth partly improves anxiety-related, but not depressive, symptoms in rats that experienced neonatal maternal separation, possibly in relation with improved function of the hypothalamic-pituitary-adrenal (HPA) axis.
Keyword
MeSH Terms
Figure
Reference
-
1. Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000; 122:81–103.2. El Khoury A, Gruber SH, Mork A, Mathe AA. Adult life behavioral consequences of early maternal separation are alleviated by escitalopram treatment in a rat model of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006; 30:535–540.3. Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002; 73:131–140.4. Daniels WM, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004; 19:3–14.5. Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996; 137:1212–1218.6. van Oers HJ, de Kloet ER, Levine S. Early vs. late maternal deprivation differentially alters the endocrine and hypothalamic responses to stress. Brain Res Dev Brain Res. 1998; 111:245–252.7. Vazquez DM, Lopez JF, Van Hoers H, Watson SJ, Levine S. Maternal deprivation regulates serotonin 1A and 2A receptors in the infant rat. Brain Res. 2000; 855:76–82.8. Jahng JW. An animal model of eating disorders associated with stressful experience in early life. Horm Behav. 2011; 59:213–220.9. Desmet PM, Schifferstein HN. Sources of positive and negative emotions in food experience. Appetite. 2008; 50:290–301.10. Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007; 61:1021–1029.11. Buwalda B, Blom WA, Koolhaas JM, van Dijk G. Behavioral and physiological responses to stress are affected by high-fat feeding in male rats. Physiol Behav. 2001; 73:371–377.12. Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004; 145:3754–3762.13. Prasad A, Prasad C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiol Behav. 1996; 60:1039–1042.14. Albanidou-Farmaki E, Poulopoulos AK, Epivatianos A, Farmakis K, Karamouzis M, Antoniades D. Increased anxiety level and high salivary and serum cortisol concentrations in patients with recurrent aphthous stomatitis. Tohoku J Exp Med. 2008; 214:291–296.15. Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000; 284:592–597.16. Kim HJ, Lee JH, Choi SH, Lee YS, Jahng JW. Fasting-induced increases of arcuate NPY mRNA and plasma corticosterone are blunted in the rat experienced neonatal maternal separation. Neuropeptides. 2005; 39:587–594.17. Lee JH, Kim HJ, Kim JG, Ryu V, Kim BT, Kang DW, Jahng JW. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci Res. 2007; 58:32–39.18. Ryu V, Lee JH, Yoo SB, Gu XF, Moon YW, Jahng JW. Sustained hyperphagia in adolescent rats that experienced neonatal maternal separation. Int J Obes (Lond). 2008; 32:1355–1362.19. Ryu V, Yoo SB, Kang DW, Lee JH, Jahng JW. Post-weaning isolation promotes food intake and body weight gain in rats that experienced neonatal maternal separation. Brain Res. 2009; 1295:127–134.20. Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc. 2007; 2:2538–2544.21. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977; 266:730–732.22. Ramos A, Mormede P. Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev. 1998; 22:33–57.23. Barros HM, Tannhauser SL, Tannhauser MA, Tannhauser M. The effects of GABAergic drugs on grooming behaviour in the open field. Pharmacol Toxicol. 1994; 74:339–344.24. Kalueff AV, Tuohimaa P. Contrasting grooming phenotypes in three mouse strains markedly different in anxiety and activity (129S1, BALB/c and NMRI). Behav Brain Res. 2005; 160:1–10.25. Ferre P, Fernandez-Teruel A, Escorihuela RM, Driscoll P, Corda MG, Giorgi O, Tobena A. Behavior of the Roman/Verh high- and low-avoidance rat lines in anxiety tests: relationship with defecation and self-grooming. Physiol Behav. 1995; 58:1209–1213.26. Nosek K, Dennis K, Andrus BM, Ahmadiyeh N, Baum AE, Solberg Woods LC, Redei EE. Context and strain-dependent behavioral response to stress. Behav Brain Funct. 2008; 4:23.27. Kalueff AV, Tuohimaa P. Grooming analysis algorithm for neurobehavioural stress research. Brain Res Brain Res Protoc. 2004; 13:151–158.28. Kompagne H, Bardos G, Szenasi G, Gacsalyi I, Harsing LG, Levay G. Chronic mild stress generates clear depressive but ambiguous anxiety-like behaviour in rats. Behav Brain Res. 2008; 193:311–314.29. Piato AL, Detanico BC, Jesus JF, Lhullier FL, Nunes DS, Elisabetsky E. Effects of Marapuama in the chronic mild stress model: further indication of antidepressant properties. J Ethnopharmacol. 2008; 118:300–304.30. Yalcin I, Aksu F, Bodard S, Chalon S, Belzung C. Antidepressant-like effect of tramadol in the unpredictable chronic mild stress procedure: possible involvement of the noradrenergic system. Behav Pharmacol. 2007; 18:623–631.31. Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011; 73:114–126.32. Garcia de Miguel B, Nutt DJ, Hood SD, Davies SJ. Elucidation of neurobiology of anxiety disorders in children through pharmacological challenge tests and cortisol measurements: a systematic review. J Psychopharmacol. 2012; 26:431–442.33. Dietrich A, Ormel J, Buitelaar JK, Verhulst FC, Hoekstra PJ, Hartman CA. Cortisol in the morning and dimensions of anxiety, depression, and aggression in children from a general population and clinic-referred cohort: an integrated analysis. The TRAILS study. Psychoneuroendocrinology. 2013; 38:1281–1298.34. Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006; 1094:202–214.35. Romeo RD, Waters EM, McEwen BS. Steroid-induced hippocampal synaptic plasticity: sex differences and similarities. Neuron Glia Biol. 2004; 1:219–229.36. Goldman L, Winget C, Hollingshead GW, Levine S. Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology. 1973; 12:199–211.37. Vazquez DM, Morano MI, Lopez JF, Watson SJ, Akil H. Short-term adrenalectomy increases glucocorticoid and mineralocorticoid receptor mRNA in selective areas of the developing hippocampus. Mol Cell Neurosci. 1993; 4:455–471.38. Boukouvalas G, Antoniou K, Papalexi E, Kitraki E. Post weaning high fat feeding affects rats' behavior and hypothalamic pituitary adrenal axis at the onset of puberty in a sexually dimorphic manner. Neuroscience. 2008; 153:373–382.39. Boukouvalas G, Gerozissis K, Markaki E, Kitraki E. High-fat feeding influences the endocrine responses of pubertal rats to an acute stress. Neuroendocrinology. 2010; 92:235–245.40. Soulis G, Papalexi E, Kittas C, Kitraki E. Early impact of a fat-enriched diet on behavioral responses of male and female rats. Behav Neurosci. 2007; 121:483–490.41. Saiyudthong S, Marsden CA. Acute effects of bergamot oil on anxiety-related behaviour and corticosterone level in rats. Phytother Res. 2011; 25:858–862.42. Scharf SH, Sterlemann V, Liebl C, Muller MB, Schmidt MV. Chronic social stress during adolescence: interplay of paroxetine treatment and ageing. Neuropharmacology. 2013; 72:38–46.43. Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002; 137:3–25.44. Di Chiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry. 1999; 46:1624–1633.45. Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci. 2008; 10:291–299.46. Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res. 1992; 577:194–199.47. Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003; 37:577–582.48. Jahng JW, Ryu V, Yoo SB, Noh SJ, Kim JY, Lee JH. Mesolimbic dopaminergic activity responding to acute stress is blunted in adolescent rats that experienced neonatal maternal separation. Neuroscience. 2010; 171:144–152.49. Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988; 42:1705–1712.50. Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003; 19:1709–1715.51. Carlezon WA Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009; 56:Suppl 1. 122–132.52. Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009; 66:1361–1372.53. Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011; 471:358–362.54. Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002; 26:321–352.55. Rezayof A, Zarrindast MR, Sahraei H, Haeri-Rohani AH. Involvement of dopamine D2 receptors of the central amygdala on the acquisition and expression of morphine-induced place preference in rat. Pharmacol Biochem Behav. 2002; 74:187–197.56. Thiel KJ, Wenzel JM, Pentkowski NS, Hobbs RJ, Alleweireldt AT, Neisewander JL. Stimulation of dopamine D2/D3 but not D1 receptors in the central amygdala decreases cocaine-seeking behavior. Behav Brain Res. 2010; 214:386–394.57. Guarraci FA, Frohardt RJ, Young SL, Kapp BS. A functional role for dopamine transmission in the amygdala during conditioned fear. Ann N Y Acad Sci. 1999; 877:732–736.58. Silberman Y, Winder DG. Corticotropin releasing factor and catecholamines enhance glutamatergic neurotransmission in the lateral subdivision of the central amygdala. Neuropharmacology. 2013; 70:316–323.59. Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem. 2004; 81:67–74.60. Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000; 861:288–295.61. Shepard JD, Barron KW, Myers DA. Stereotaxic localization of corticosterone to the amygdala enhances hypothalamo-pituitary-adrenal responses to behavioral stress. Brain Res. 2003; 963:203–213.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experience of Neonatal Maternal Separation May Lead to a Long-term Modulation in the Neuronal Activity of Nucleus Accumbens in the Offspring
- Evaluation of function and disorders of the adrenal gland in neonates
- Effects of oropharyngeal taste stimuli in the restoration of the fasting-induced activation of the HPA axis in rats
- Chronic administration of ketamine ameliorates the anxiety- and aggressive-like behavior in adolescent mice induced by neonatal maternal separation
- Hypothalamic-Pituitary-Adrenal Axis and Epilepsy