Endocrinol Metab.
2014 Jun;29(2):163-168. 10.3803/EnM.2014.29.2.163.
Insulin Phosphorylates Tyrosine Residue 464 of Tub and Translocates Tubby into the Nucleus in HIRcB Cells
- Affiliations
-
- 1Department of Neurosurgery, Gachon University Gil Medical Center, Incheon, Korea.
- 2Department of Anatomy, Korea University College of Medicine, Seoul, Korea.
- 3Department of Neurosurgery, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
- 4Department of Neurosurgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea. jypark@kumc.or.kr
- KMID: 2169423
- DOI: http://doi.org/10.3803/EnM.2014.29.2.163
Abstract
- BACKGROUND
The tubby protein has a motif that might be relevant for its action in the insulin signaling pathway. Previous studies have indicated that tubby undergoes phosphorylation on tyrosine residues in response to several stimuli and is known to localize in the nucleus as well as in the plasma membrane. However, the relationship between phosphorylation and nuclear translocation is not well understood. Here, we report that insulin directly phosphorylates tubby, which translocates into the nucleus.
METHODS
The effects of insulin on Tubby were performed with Western blot. The immunoprecipitation and confocal microscopy were performed to prove phosphorylation and nuclear translocation.
RESULTS
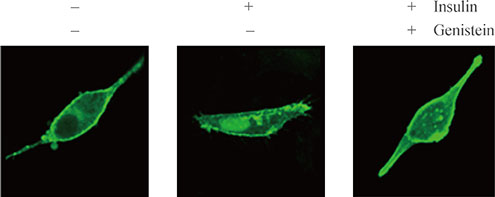
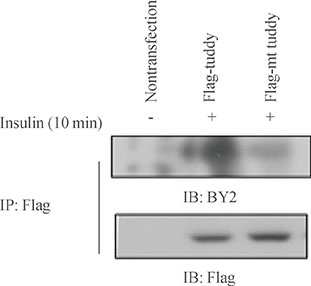
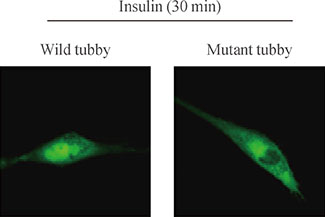
Mutation study reveals that tyrosine residue 464 of tubby gene (Tub) is a phosphorylation site activated by insulin. In addition, major portions of tubby protein in the plasma membrane are translocated into the nucleus after insulin treatment. Tyrosine kinase inhibitor pretreatment blocked insulin-induced tubby translocation, suggesting that phosphorylation is important for nuclear translocation. Moreover, mutant tyrosine residue 464 did not translocate into the nucleus in respond to insulin. These findings demonstrate that insulin phosphorylates tyrosine residue 464 of Tub, and this event is important for insulin-induced tubby nuclear translocation.
CONCLUSION
Insulin phosphorylates tyrosine residue 464 of Tub and translocates tubby into the nuclei of HIRcB cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Carroll K, Gomez C, Shapiro L. Tubby proteins: the plot thickens. Nat Rev Mol Cell Biol. 2004; 5:55–63.2. Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003; 421:268–272.3. Ronshaugen M, McGinnis N, Inglis D, Chou D, Zhao J, McGinnis W. Structure and expression patterns of Drosophila TULP and TUSP, members of the tubby-like gene family. Mech Dev. 2002; 117:209–215.4. Ikeda A, Naggert JK, Nishina PM. Genetic modification of retinal degeneration in tubby mice. Exp Eye Res. 2002; 74:455–461.5. Ikeda A, Zheng QY, Zuberi AR, Johnson KR, Naggert JK, Nishina PM. Microtubule-associated protein 1A is a modifier of tubby hearing (moth1). Nat Genet. 2002; 30:401–405.6. Grant SG. Putting tubby on the MAP. Nat Genet. 2002; 30:347–348.7. Ikeda A, Nishina PM, Naggert JK. The tubby-like proteins, a family with roles in neuronal development and function. J Cell Sci. 2002; 115(Pt 1):9–14.8. Li QZ, Wang CY, Shi JD, Ruan QG, Eckenrode S, Davoodi-Semiromi A, Kukar T, Gu Y, Lian W, Wu D, She JX. Molecular cloning and characterization of the mouse and human TUSP gene, a novel member of the tubby superfamily. Gene. 2001; 273:275–284.9. Heikenwalder MF, Koritschoner NP, Pajer P, Chaboissier MC, Kurz SM, Briegel KJ, Bartunek P, Zenke M. Molecular cloning, expression and regulation of the avian tubby-like protein 1 (tulp1) gene. Gene. 2001; 273:131–139.10. Hagstrom SA, Adamian M, Scimeca M, Pawlyk BS, Yue G, Li T. A role for the Tubby-like protein 1 in rhodopsin transport. Invest Ophthalmol Vis Sci. 2001; 42:1955–1962.11. Koritschoner NP, Alvarez-Dolado M, Kurz SM, Heikenwalder MF, Hacker C, Vogel F, Munoz A, Zenke M. Thyroid hormone regulates the obesity gene tub. EMBO Rep. 2001; 2:499–504.12. Cantley LC. Transcription. Translocating tubby. Science. 2001; 292:2019–2021.13. Ikeda A, Ikeda S, Gridley T, Nishina PM, Naggert JK. Neural tube defects and neuroepithelial cell death in Tulp3 knockout mice. Hum Mol Genet. 2001; 10:1325–1334.14. Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS, Myszka DG, Shapiro L. G-protein signaling through tubby proteins. Science. 2001; 292:2041–2050.15. He W, Ikeda S, Bronson RT, Yan G, Nishina PM, North MA, Naggert JK. GFP-tagged expression and immunohistochemical studies to determine the subcellular localization of the tubby gene family members. Brain Res Mol Brain Res. 2000; 81:109–117.16. Kapeller R, Moriarty A, Strauss A, Stubdal H, Theriault K, Siebert E, Chickering T, Morgenstern JP, Tartaglia LA, Lillie J. Tyrosine phosphorylation of tub and its association with Src homology 2 domain-containing proteins implicate tub in intracellular signaling by insulin. J Biol Chem. 1999; 274:24980–24986.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autophosphorylation and tyrosine kinase activity of purified insulin receptors
- Microarray Analysis of Differentially Expressed Genes in the Brains of Tubby Mice
- Detection of the amplified tyrosine kinase domain in the insulin receptor beta-subunit by the PCR method
- The regulatory mechanism of phosphatidylinositol 3-kinase by insulin in 3T3 L1 fibroblasts: phosphorylation-independent activation of phosphatidylinositol 3-kinase
- Effect of Articular Immobilization - Induced Hindlimb Skeletal Muscle Atrophy on Autophosphorylation of Tyrosine Kinase of the Insulin Receptor in Rats