J Bacteriol Virol.
2011 Jun;41(2):117-122. 10.4167/jbv.2011.41.2.117.
Investigation of Murine Norovirus Replication in RAW264.7 Cells by Strand-specific RT-PCR
- Affiliations
-
- 1Department of Microbiology, Chungbuk National University, Cheongju, Korea. chlee@cbu.ac.kr
- 2Department of Microbiology, Catholic University Medical School, Seoul, Korea.
- 3School of Public Health, Seoul National University, Seoul, Korea.
- 4National Institute of Environmental Research, Incheon, Korea.
- KMID: 2168629
- DOI: http://doi.org/10.4167/jbv.2011.41.2.117
Abstract
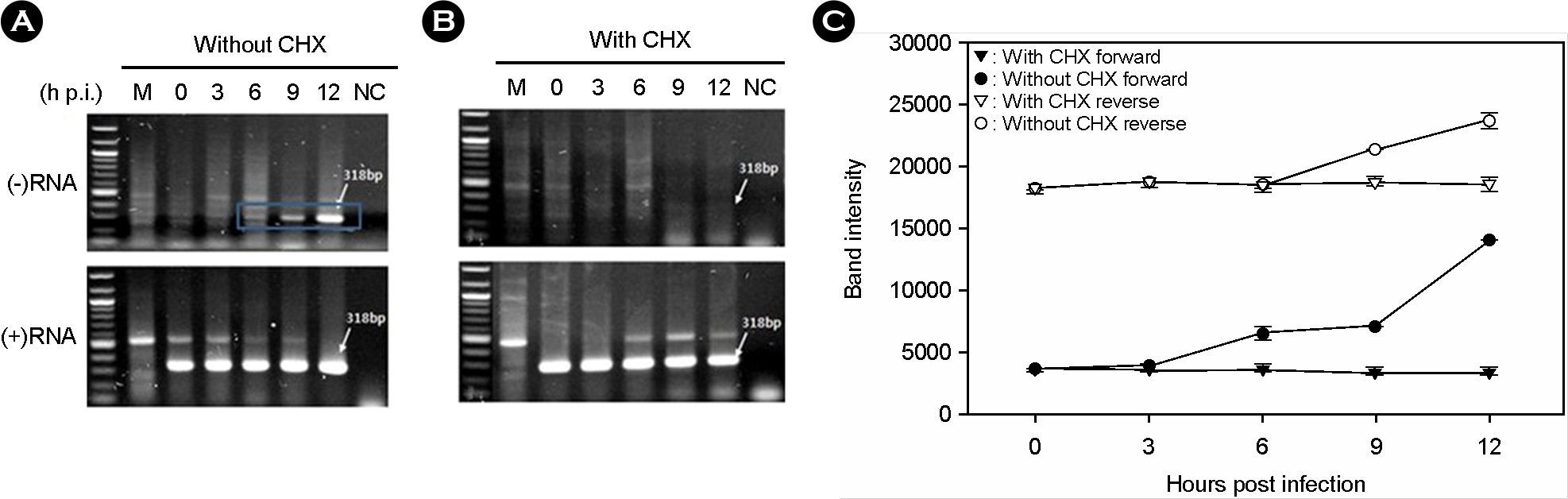
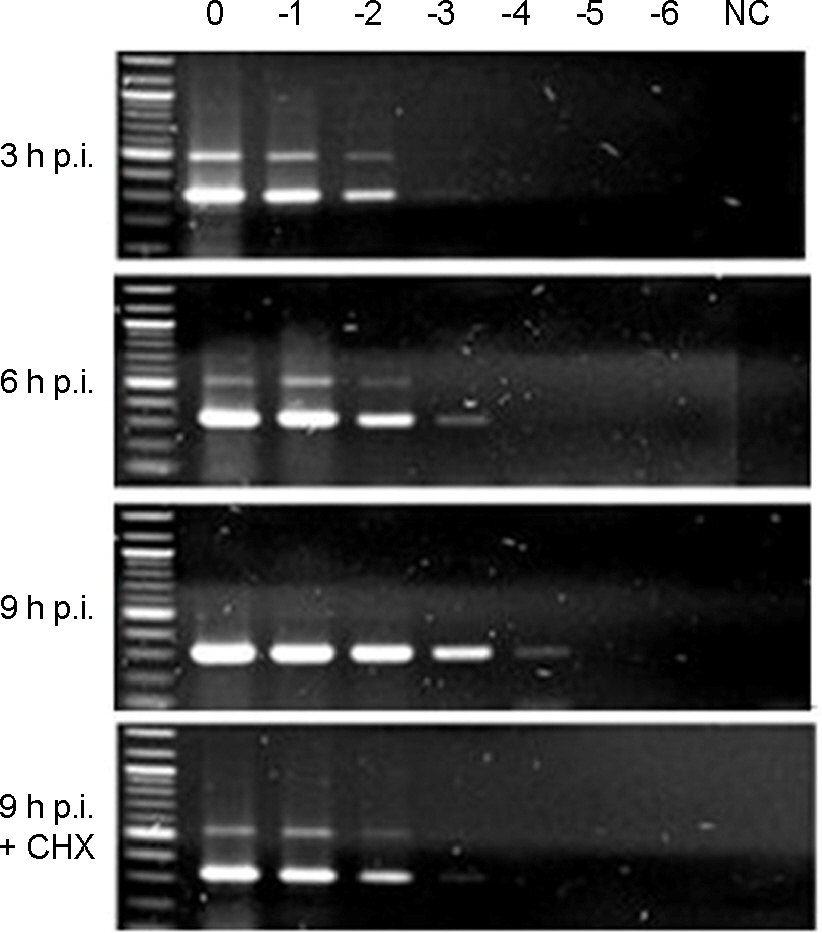
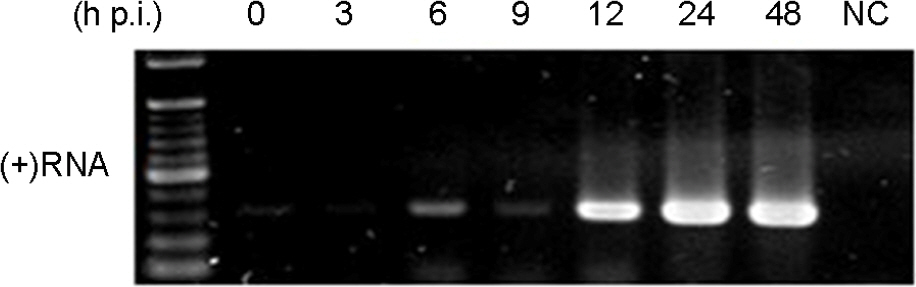
- Murine norovirus (MNV) is a non-enveloped virus with a positive-sense RNA genome and causes lethal infection in mice. MNV has been used as a model virus for human norovirus (NV) whose in vitro cell culture system has not been available to date since MNV and NV are genetically related. In this study, the genome replication of MNV was investigated using strand-specific RT-PCR in RAW264.7 cells. Reverse transcription (RT) using a sense primer followed by PCR showed that negative-sense RNAs were first detected in RAW264.7 cells between 6 and 9 [3 and 6] hours post infection (h.p.i.). However, these negative-sense RNAs were not detected when cells were treated with a translation inhibitor cycloheximide. Then, RT with an antisense primer followed by PCR was performed to detect positive-sense RNAs. RT-PCR results revealed that the amount of positive-sense RNAs began to increase from 9 [6] h.p.i., indicating the accumulation of the newly synthesized (+)RNA genome. Furthermore, cycloheximide abrogated the increase of newly made RNAs during MNV infection. In conclusion, strand-specific RT-PCR using a sense or antisense primer, in combination with cycloheximide treatment, enabled us to detect positive-sense and negative-sense RNAs selectively and provided a useful tool to understand the replication cycle of MNV.
MeSH Terms
Figure
Reference
-
1). Fankhauser RL., Monroe SS., Noel JS., Humphrey CD., Bresee JS., Parashar UD, et al. Epidemiologic and molecular trends of Norwalk-like viruses associated with outbreaks of gastroenteritis in the United States. J Infect Dis. 2002. 186:1–7.
Article2). Inouye S., Yamashita K., Yamadera S., Yoshikawa M., Kato N., Okabe N. Surveillance of viral gastroenteritis in Japan: pediatric cases and outbreak incidents. J Infect Dis. 2000. 181:S270–4.
Article3). Lopman BA., Reacher MH., Van Duijnhoven Y., Hanon FX., Brown D., Koopmans M. Viral gastroenteritis outbreaks in Europe, 1995-2000. Emerg Infect Dis. 2003. 9:90–6.
Article4). Rockx B., De Wit M., Vennema H., Vinjé J., De Bruin E., van Duynhoven Y, et al. Natural history of Human Calicivirus infection: a prospective cohort study. Clin Infect Dis. 2002. 35:246–53.5). Zheng DP., Ando T., Fankhauser RL., Beard RS., Glass RI., Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006. 346:312–23.
Article6). Adler JL., Zickl R. Winter vomiting disease. J Infect Dis. 1969. 119:668–73.
Article7). Kapikian AZ., Wyatt RG., Dolin R., Thornhill TS., Kalica AR., Chanock RM. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972. 10:1075–81.
Article8). Xi JN., Graham DY., Wang KN., Estes MK. Norwalk virus genome cloning and characterization. Science. 1990. 250:1580–3.
Article9). Duizer E., Schwab KJ., Neill FH., Atmar RL., Koopmans MP., Estes MK. Laboratory efforts to cultivate noroviruses. J Gen Virol. 2004. 85:79–87.
Article10). Straub TM., Höner zu Bentrup K., Orosz-Coghlan P., Dohnalkova A., Mayer BK., Bartholomew RA, et al. In vitro cell culture infectivity assay for human noroviruses. Emerg Infect Dis. 2007. 13:396–403.11). Wobus CE., Karst SM., Thackray LB., Chang KO., Sosnovtsev SV., Belliot G, et al. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004. 2:e432.
Article12). Wobus CE., Thackray LB., Virgin HW 4th. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol. 2006. 80:5104–12.
Article13). Karst SM., Wobus CE., Lay M., Davidson J., Virgin HW 4th. STAT1-dependent innate immunity to a Norwalk-like virus. Science. 2003. 299:1575–8.
Article14). Craggs JK., Ball JK., Thomson BJ., Irving WL., Grabowska AM. Development of a strand-specific RT-PCR based assay to detect the replicative form of hepatitis C virus RNA. J Virol Methods. 2001. 94:111–20.
Article15). Lin L., Fevery J., Hiem Yap S. A novel strand-specific RT-PCR for detection of hepatitis C virus negative-strand RNA (replicative intermediate): evidence of absence or very low level of HCV replication in peripheral blood mononuclear cells. J Virol Methods. 2002. 100:97–105.
Article16). Liu HS., Lin YL., Chen CC. Comparison of various methods of detection of different forms of dengue virus type 2 RNA in cultured cells. Acta Virol. 1997. 41:317–24.17). Vaughan G., Olivera H., Santos-Argumedo L., Landa A., Briseño B., Escobar-Gutiérrez A. Dengue virus replicative intermediate RNA detection by reverse transcription-PCR. Clin Diagn Lab Immunol. 2002. 9:198–200.
Article18). Jang SY., Jeong WH., Kim MS., Lee YM., Lee JI., Lee GC, et al. Detection of replicating negative-sense RNAs in CaCo-2 cells infected with human astrovirus. Arch Virol. 2010. 155:1383–9.
Article19). Ali N., Tardif KD., Siddiqui A. Cell-free replication of the hepatitis C virus subgenomic replicon. J Virol. 2002. 76:12001–7.
Article20). Bartholomeusz AI., Wright PJ. Synthesis of dengue virus RNA in vitro: initiation and the involvement of proteins NS3 and NS5. Arch Virol. 1993. 128:111–21.21). Barton DJ., Black EP., Flanegan JB. Complete replication of poliovirus in vitro: preinitiation RNA replication complexes require soluble cellular factors for the synthesis of VPg-linked RNA. J Virol. 1995. 69:5516–27.22). Chu PW., Westaway EG. Replication strategy of Kunjin virus: evidence for recycling role of replicative form RNA as template in semiconservative and asymmetric replication. Virology. 1985. 140:68–79.
Article23). Gu C., Zheng C., Shi L., Zhang Q., Li Y., Lu B, et al. Plus- and minus-stranded foot-and-mouth disease virus RNA quantified simultaneously using a novel real-time RT-PCR. Virus Genes. 2007. 34:289–98.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Validation of RT-PCR Assay for Detection of Replication-competent Lentivirus (RCL) in Vector Preparations Using HIV Vector Based Gene Delivery System
- Human Norovirus Genogroups Detected from Acute Gastroenteritis Patients in Seoul from May 2013 to April 2015
- Vinyl-Stilbene Inhibits Human Norovirus RNA Replication by Activating Heat-Shock Factor-1
- Recovery and Adsorption Rate of Murine Norovirus Using NanoCeram(R) Filters
- TNF-alpha and IL-12 Secretion in Macrophages in Response to Spores of Bacillus anthracis Sterne