J Bacteriol Virol.
2011 Jun;41(2):109-116. 10.4167/jbv.2011.41.2.109.
A Sequence in Coat Protein Open Reading Frame Is Required for Turnip Yellow Mosaic Virus Replication
- Affiliations
-
- 1Department of Biochemistry, Chungbuk National University, Cheongju, Korea. tjcho@chungbuk.ac.kr
- KMID: 2168628
- DOI: http://doi.org/10.4167/jbv.2011.41.2.109
Abstract
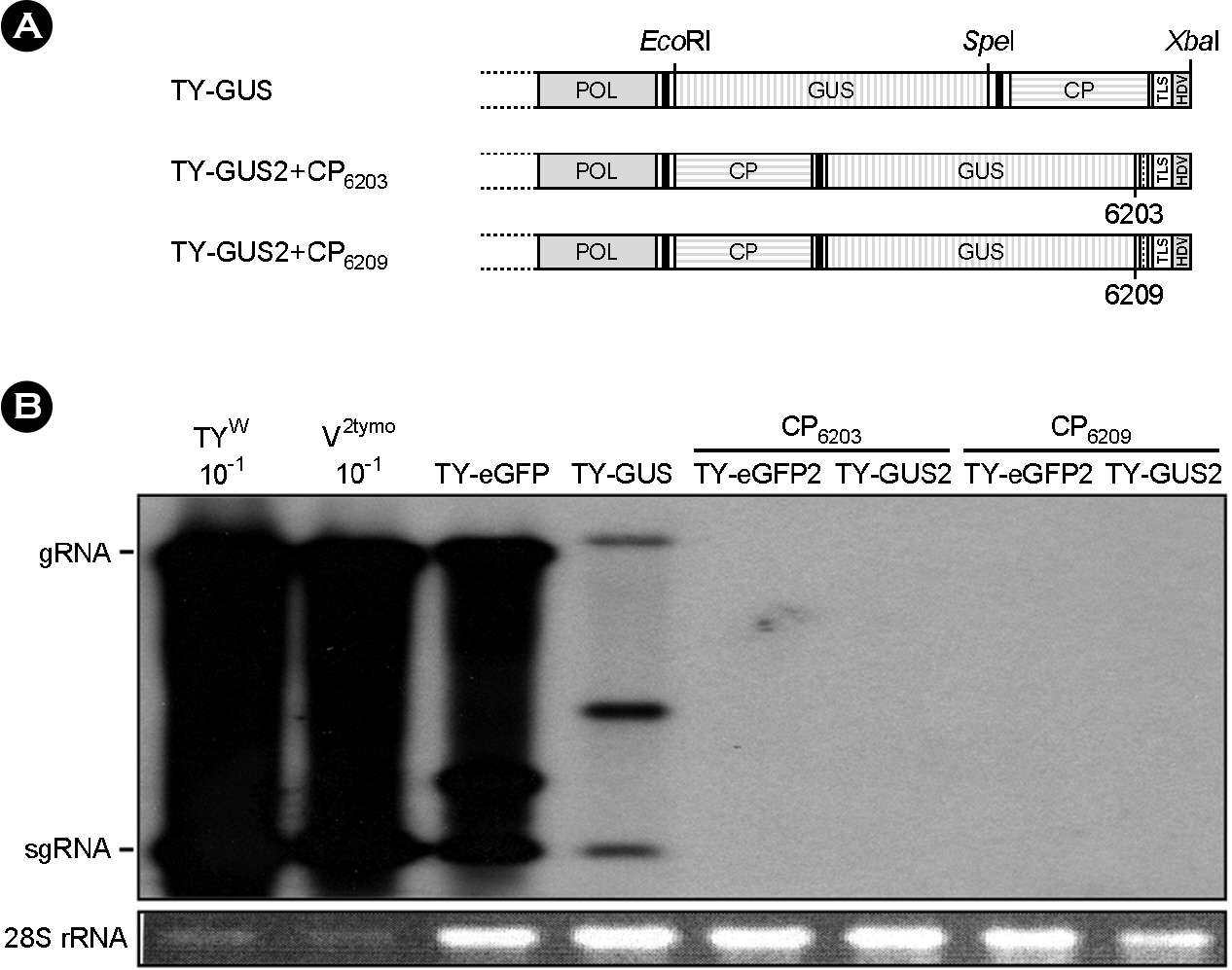
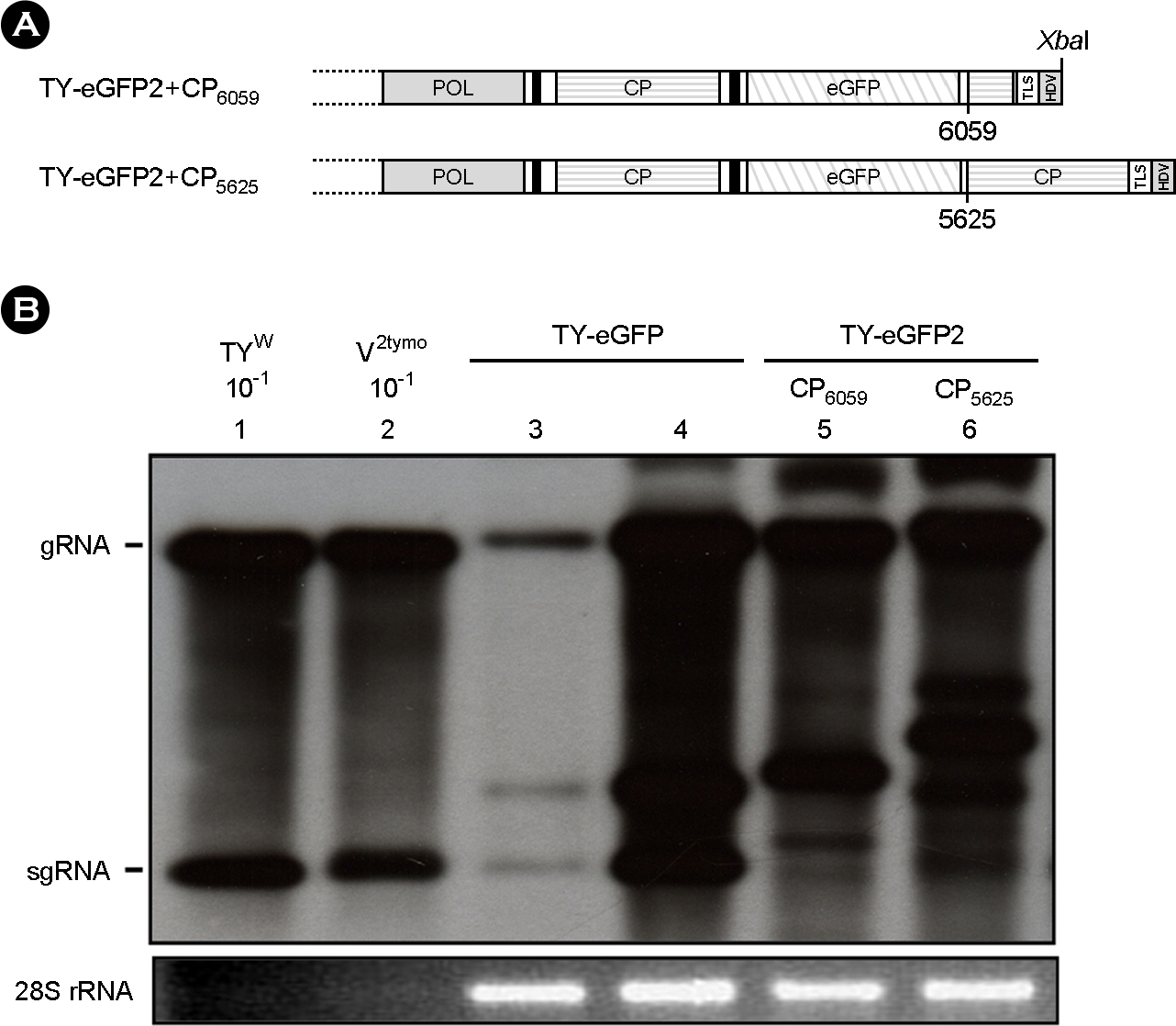
- Turnip yellow mosaic virus (TYMV) is a spherical plant virus that has a single 6.3 kb positive strand RNA genome. Information for TYMV replication is limited, except that the 3'-terminal sequence and 5'-untranslated region are required for genome replication. When a foreign sequence was inserted at the position upstream of the coat protein (CP) open reading frame (ORF), replication of the recombinant TYMV was comparable to wild type, as long as an RNAi suppressor was provided. In contrast, when the foreign sequence was inserted between the CP ORF and the 3'-terminal tRNA-like structure, replication of the recombinant virus was not detected. This result suggests that the CP ORF contains an essential replication element which should be appropriately spaced with respect to the 3'-end. Analysis of TYMV constructs containing a part or a full additional CP ORF indicates that the 3' quarter of the CP ORF is required for TYMV replication.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Characterization of a Replication Element in the Coat Protein ORF of Turnip Yellow Mosaic Virus
Hyun-Il Shin, Tae-Ju Cho
J Bacteriol Virol. 2012;42(1):49-55. doi: 10.4167/jbv.2012.42.1.49.Read-through Mutation in the Coat Protein ORF Suppresses Turnip Yellow Mosaic Virus Subgenomic RNA Accumulation
Hyun-Il Shin, Tae-Ju Cho
J Bacteriol Virol. 2013;43(1):54-63. doi: 10.4167/jbv.2013.43.1.54.Cysteine-Added Mutants of Turnip Yellow Mosaic Virus
In-Sun Shin, Doyeong Kim, Tae-Ju Cho
J Bacteriol Virol. 2018;48(4):137-146. doi: 10.4167/jbv.2018.48.4.137.
Reference
-
1). Dreher TW. Turnip yellow mosaic virus: transfer RNA mimicry, chloroplasts and a C-rich genome. Mol Plant Pathol. 2004. 5:367–75.
Article2). Bozarth CS., Weiland JJ., Dreher TW. Expression of ORF-69 of turnip yellow mosaic virus is necessary for viral spread in plants. Virology. 1992. 187:124–30.
Article3). Tsai CH., Dreher TW. Increased viral yield and symptom severity result from a single amino acid substitution in the turnip yellow mosaic virus movement protein. Mol Plant Microbe Interact. 1993. 6:268–73.
Article4). Chen J., Li WX., Xie D., Peng JR., Ding SW. Viral virulence protein suppresses RNA silencing-mediated defense but upregulates the role of microRNA in host gene expression. Plant Cell. 2004. 16:1302–13.5). Dreher TW., Goodwin JB. Transfer RNA mimicry among tymoviral genomic RNAs ranges from highly efficient to vestigial. Nucleic Acids Res. 1998. 26:4356–64.
Article6). Dreher TW., Tsai CH., Florentz C., Giegé R. Specific valylation of turnip yellow mosaic virus RNA by wheat germ valyltRNA synthetase determined by three anticodon loop nucleotides. Biochemistry. 1992. 31:9183–9.
Article7). Matsuda D., Dreher TW. The tRNA-like structure of Turnip yellow mosaic virus RNA is a 3′-translational enhancer. Virology. 2004. 321:36–46.
Article8). Miller WA., Bujarski JJ., Dreher TW., Hall TC. Minus-strand initiation by brome mosaic virus replicase within the 3′ tRNA-like structure of native and modified RNA templates. J Mol Biol. 1986. 187:537–46.
Article9). Deiman BA., Koenen AK., Verlaan PW., Pleij CW. Minimal template requirements for initiation of minus-strand synthesis in vitro by the RNA-dependent RNA polymerase of turnip yellow mosaic virus. J Virol. 1998. 72:3965–72.10). Shin HI., Cho NJ., Cho TJ. Role of 5′-UTR hairpins of the Turnip yellow mosaic virus RNA in replication and systemic movement. BMB Rep. 2008. 41:778–83.
Article11). Shin HI., Tzanetakis IE., Dreher TW., Cho TJ. The 5′-UTR of Turnip yellow mosaic virus does not include a critical encapsidation signal. Virology. 2009. 387:427–35.
Article12). Shin HI., Kim IC., Cho TJ. Replication and encapsidation of recombinant Turnip yellow mosaic virus RNA. BMB Rep. 2008. 41:739–44.
Article13). Cho TJ., Dreher TW. Encapsidation of genomic but not sub-genomic turnip yellow mosaic virus RNA by coat protein provided in trans. Virology. 2006. 356:126–35.
Article14). Voinnet O., Pinto YM., Baulcombe DC. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc Natl Acad Sci. 1999. 96:14147–52.
Article15). Ray D., White KA. Enhancer-like properties of an RNA element that modulates tombusvirus RNA accumulation. Virology. 1999. 256:162–71.
Article16). White KA., Nagy PD. Advances in the molecular biology of tombusviruses: gene expression, genome replication, and recombination. Prog Nucleic Acid Res Mol Biol. 2004. 78:187–226.
Article17). Pogany J., Fabian MR., White KA., Nagy PD. A replication silencer element in a plus-strand RNA virus. EMBO J. 2003. 22:5602–11.
Article18). Ray D., Na H., White KA. Structure properties of a multifunctional T-shaped RNA domain that mediate efficient Tomato bushy stunt virus RNA replication. J Virol. 2004. 78:10490–500.19). Panaviene Z., Panavas T., Nagy PD. Role of an internal and two 3′-terminal RNA elements in assembly of tombusvirus replicase. J Virol. 2005. 79:10608–18.
Article20). Kim KH., Hemenway CL. Long-distance RNA-RNA interactions and conserved sequence elements affect potato virus X plus-strand RNA accumulation. RNA. 1999. 5:636–45.
Article21). Miller WA., White KA. Long-distance RNA-RNA interactions in plant virus gene expression and replication. Annu Rev Phytopathol. 2006. 44:447–67.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Replication of Recombinant Flock House Virus RNA Encapsidated by Turnip Yellow Mosaic Virus Coat Proteins in Nicotiana benthamiana
- Read-through Mutation in the Coat Protein ORF Suppresses Turnip Yellow Mosaic Virus Subgenomic RNA Accumulation
- Characterization of a Replication Element in the Coat Protein ORF of Turnip Yellow Mosaic Virus
- N-terminal Extension of Coat Protein of Turnip Yellow Mosaic Virus has Variable Effects on Replication, RNA Packaging, and Virion Assembly Depending on the Inserted Sequence
- Genome Size Constraint in Replication and Packaging of Turnip Yellow Mosaic Virus