J Bacteriol Virol.
2016 Mar;46(1):13-21. 10.4167/jbv.2016.46.1.13.
N-terminal Extension of Coat Protein of Turnip Yellow Mosaic Virus has Variable Effects on Replication, RNA Packaging, and Virion Assembly Depending on the Inserted Sequence
- Affiliations
-
- 1Department of Biochemistry, Chungbuk National University, Cheongju, Korea. tjcho@chungbuk.ac.kr
- KMID: 2168738
- DOI: http://doi.org/10.4167/jbv.2016.46.1.13
Abstract
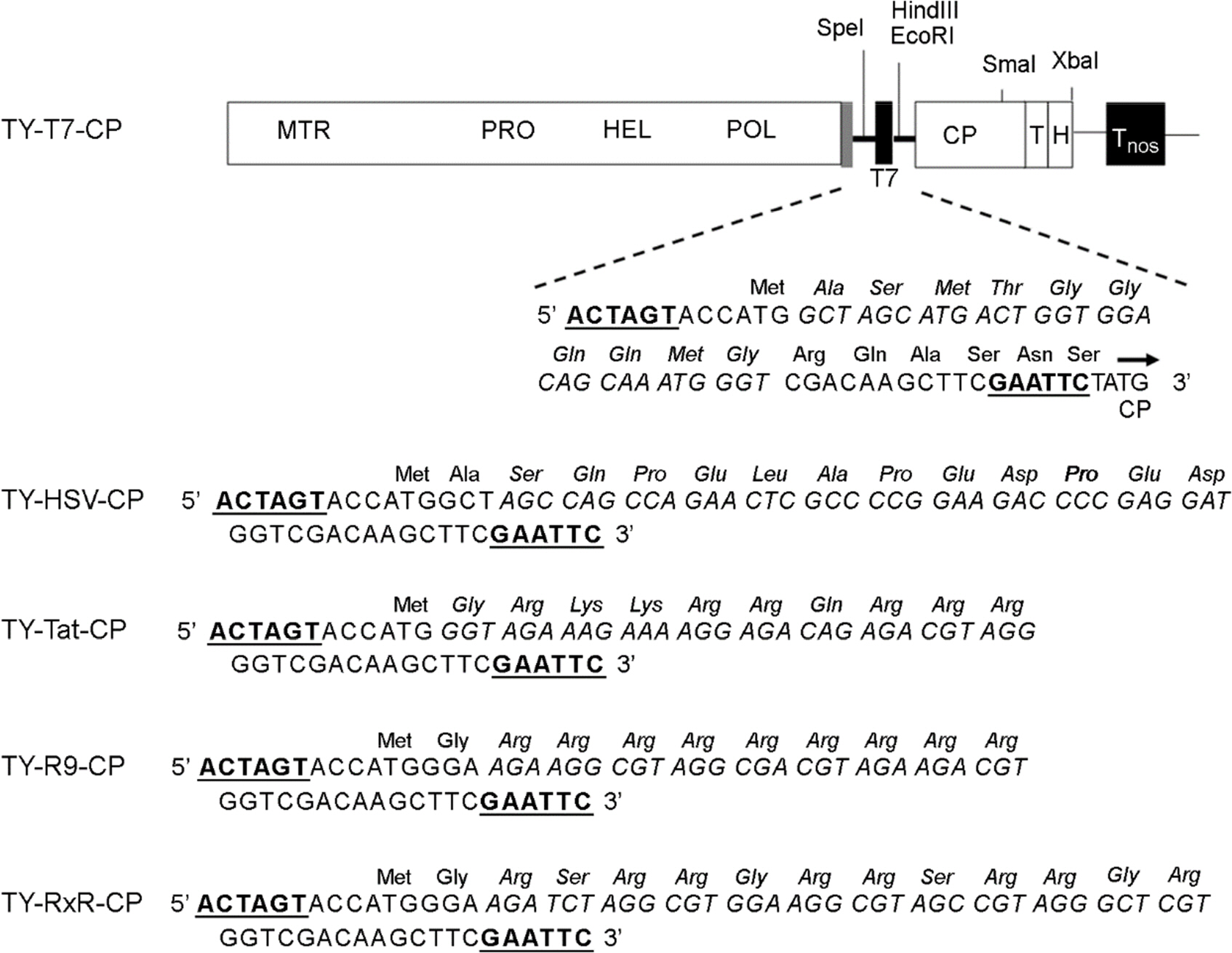
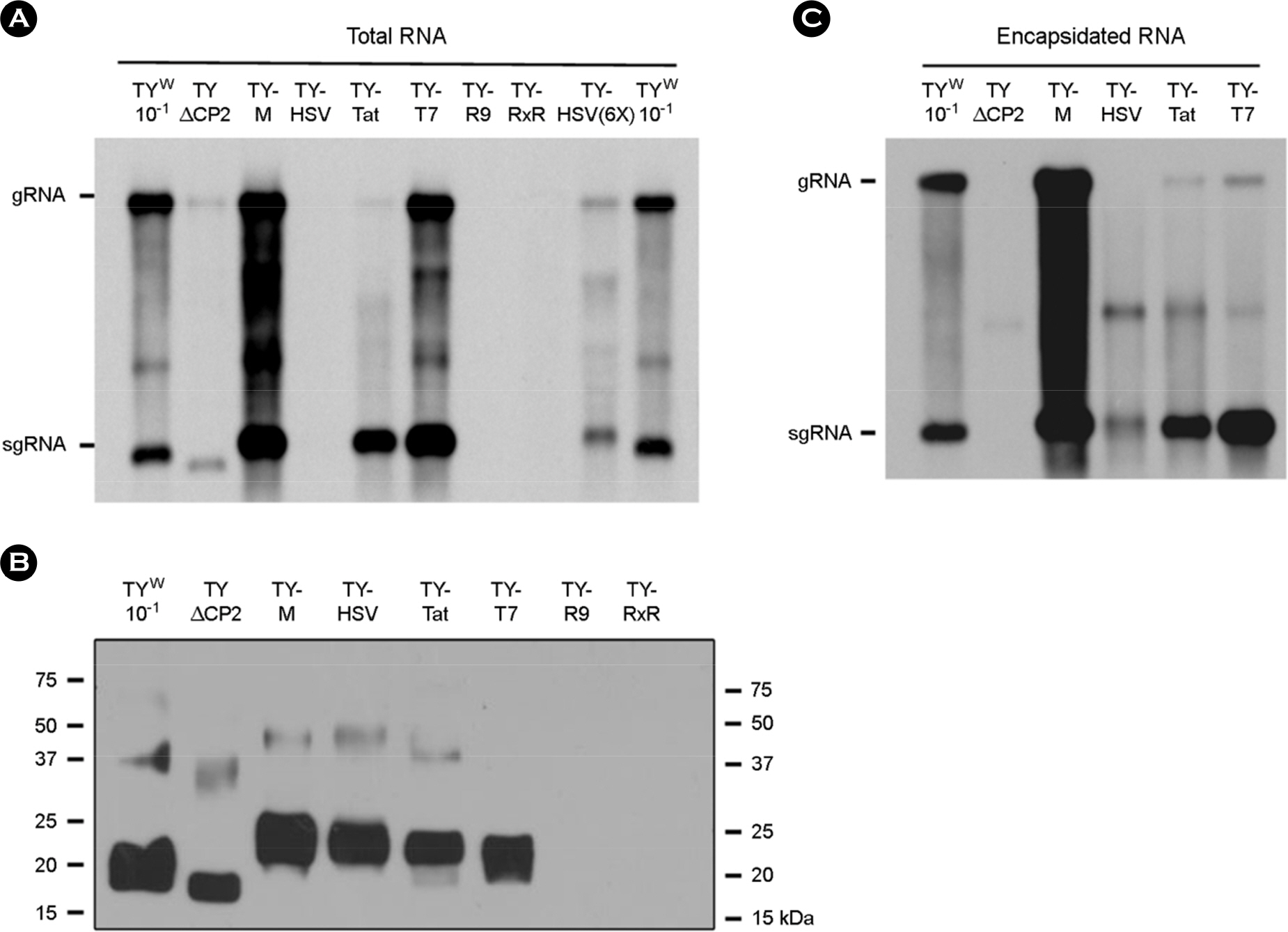
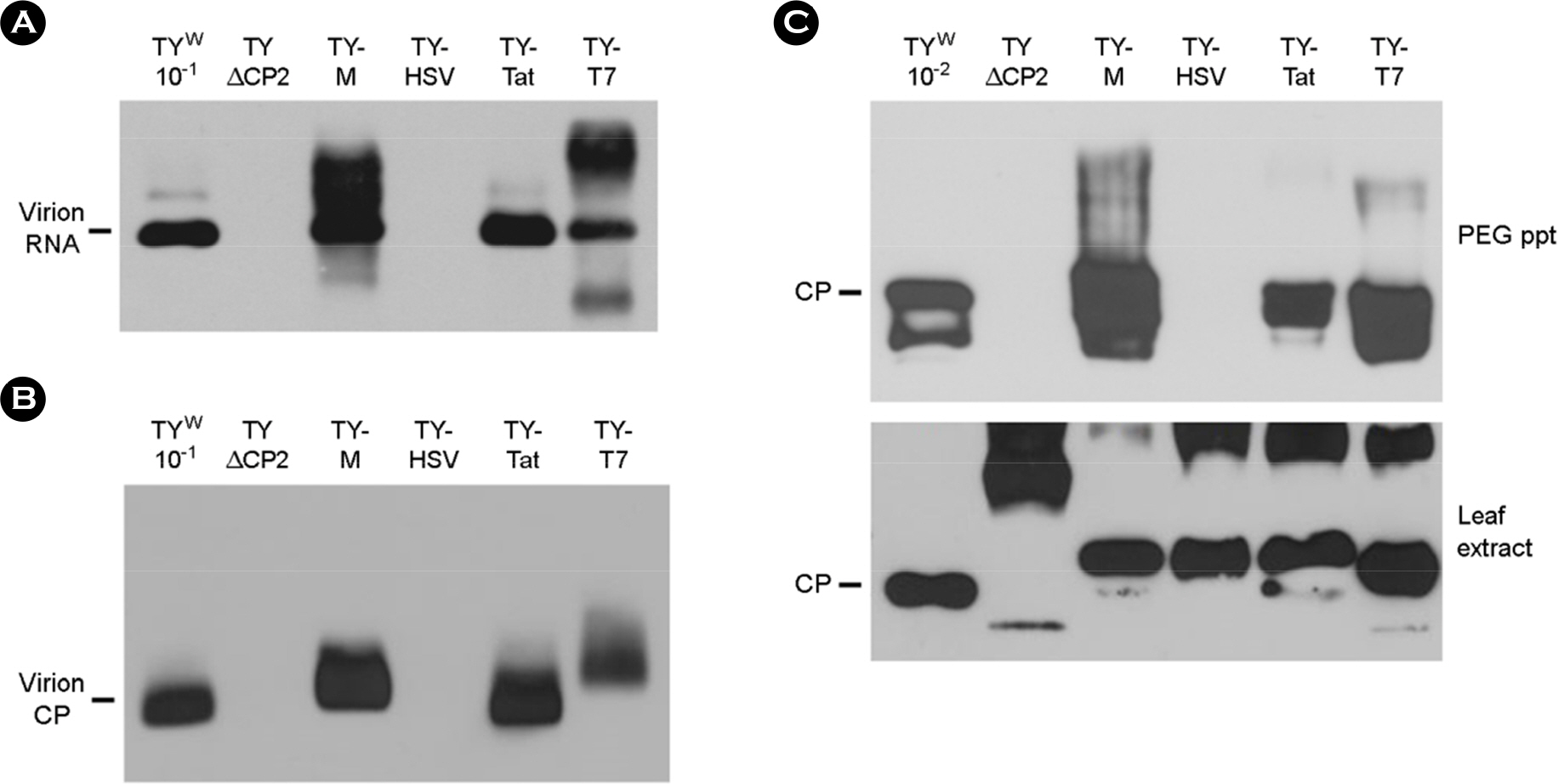
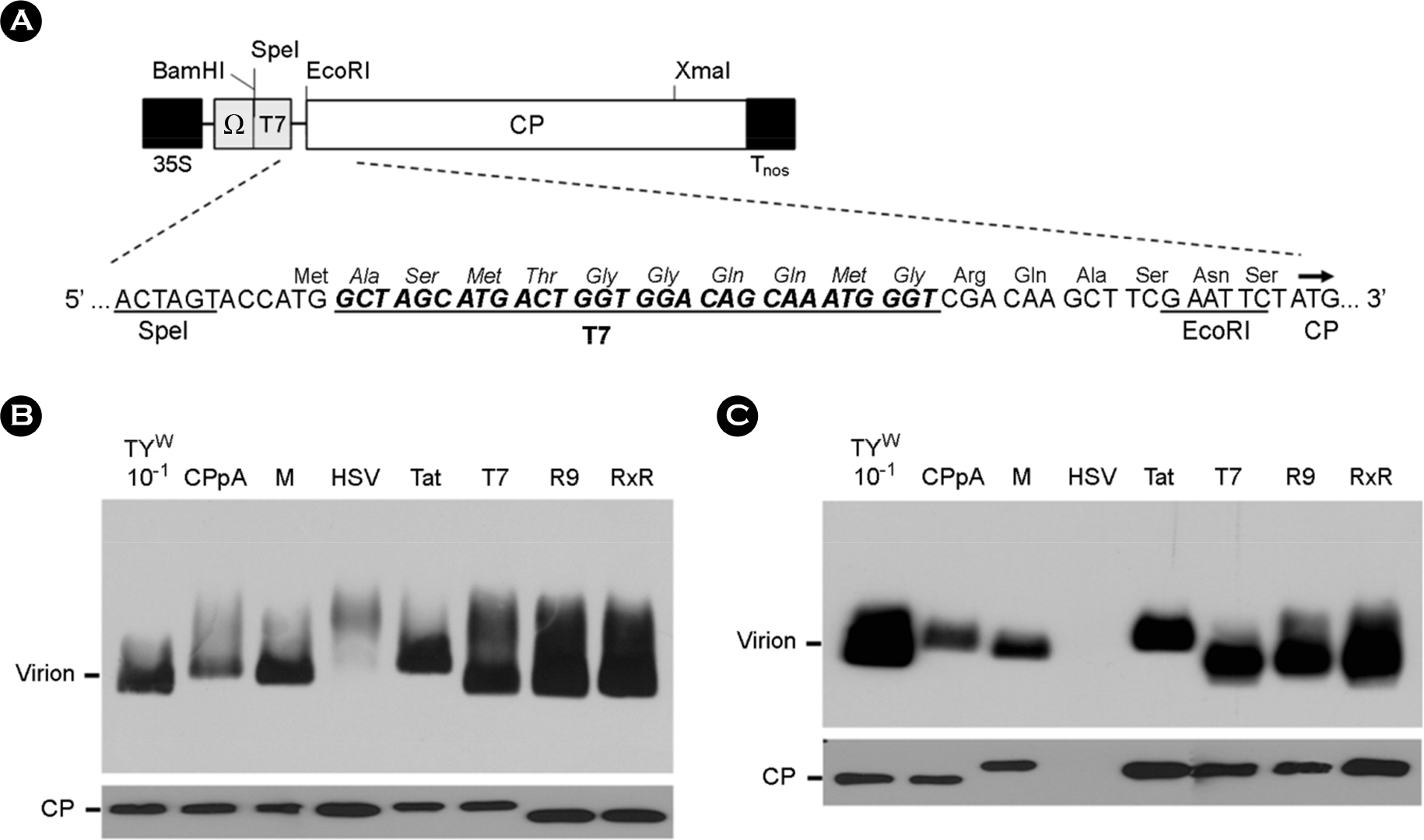
- Turnip yellow mosaic virus (TYMV) is a non-enveloped icosahedral virus composed of 20 kDa single coat proteins. In this study, we modified the TYMV coat protein (CP) ORF by inserting an oligonucleotide linker corresponding to T7, HSV, Tat, (Arg)9, or (RxR)4 peptide at the 5'-end of the CP ORF and examined its effect on replication, RNA packaging, and virion assembly. The results showed that the constructs containing (Arg)9 and (RxR)4 sequences were barely capable of replication. The TYMV constructs containing T7 and Tat peptide produced virions that co-migrated with wild-type virions. However, the insertion of T7 and Tat sequences impaired genomic RNA (gRNA) accumulation and packaging, respectively. When only the CP gene was expressed, CPs with (Arg)9 or (RxR)4 successfully produced virus-like particles whose mobility was comparable to that of wild type. In the case of CP having a HSV tag, the virion band was not detected, although a sufficient amount of CP was produced. This indicates that CP with the HSV tag failed to assemble into virions. Overall, the results suggest that TYMV replication, RNA packaging and virion assembly are strongly influenced by the insertion sequence.
Keyword
MeSH Terms
Figure
Reference
-
1). Dreher TW. Turnip yellow mosaic virus: transfer RNA mimicry, chloroplasts and a C-rich genome. Mol Plant Pathol. 2004; 5:367–75.
Article2). Canady MA, Larson SB, Day J, McPherson A. Crystal structure of turnip yellow mosaic virus. Nat Struct Biol. 1996; 3:771–81.
Article3). Steinmetz NF, Evans DJ. Utilization of plant viruses in biotechnology. Org Biomol Chem. 2007; 5:2891–902.4). Steinmetz NF. Viral nanoparticles as platforms for next-generation therapeutics and imaging devices. Nanomedicine. 2010; 6:634–41.
Article5). Yildiz I, Shukla S, Steinmetz NF. Applications of viral nanoparticles in medicine. Curr Op Biotechnol. 2011; 22:901–8.
Article6). Brundel FM, Lewis JD, Desitto G, Steinmetz NF, Manchester M, Stuhlmann H, et al. Hydrazone ligation strategy to assemble multifunctional viral nanoparticles for cell imaging and tumor targeting. Nano Lett. 2010; 10:1093–7.7). Hayden CM, Mackenzie AM, Skotnicki ML, Gibbs A. Turnip yellow mosaic virus isolates with experimentally produced recombinant virion proteins. J Gen Virol. 1998; 79:395–403.
Article8). Powel JD, Barbar E, Dreher TW. Turnip yellow mosaic virus forms infectious particles without the native beta-annulus structure and flexible coat protein N-terminus. Virology. 2012; 422:165–73.
Article9). Shin H-I, Chae K-H, Cho T-J. Modification of Turnip yellow mosaic virus coat protein and its effect on virion assembly. BMB reports. 2013; 46:495–500.
Article10). Verdurmen WPR, Brock R. Biological responses towards cationic peptides and drug carriers. Trends Pharmacol Sci. 2011; 32:116–24.
Article11). Cho T-J, Dreher TW. Encapsidation of genomic but not subgenomic Turnip yellow mosaic virus RNA by coat protein provided in trans. Virology. 2006; 356:126–35.
Article12). Lane LC. Propagation and purification of RNA plant viruses. Methods Enzymol. 1986; 118:687–9.
Article13). Shin H-I, Cho T-J. Read-through mutation in the coat protein ORF suppresses Turnip yellow mosaic virus subgenomic RNA accumulation. J Bacteriol Virol. 2013; 43:54–63.
Article14). Shin H-I, Tzanetakis IE, Dreher TW, Cho T-J. The 5′-UTR of Turnip yellow mosaic virus does not include a critical encapsidation signal. Virology. 2009; 387:427–35.
Article15). Deiman BALM, Verlaan PWG, Pleij CWA. In vitro transcription by the turnip yellow mosaic virus RNA polymerase: a comparison with the alfalfa mosaic virus and brome mosaic virus replicases. J Virol. 2000; 74:264–71.16). Singh RN, Dreher TW. Specific site selection in RNA resulting from a combination of nonspecific secondary structure and -CCRboxes: initiation of minus strand synthesis by turnip yellow mosaic virus RNA-dependent RNA polymerase. RNA. 1998; 4:1083–95.17). Colussi TM, Costantino DA, Hammond JA, Ruehle GM, Nix JC, Kieft JS. The structural basis of transfer RNA mimicry and conformational plasticity by a viral RNA. Nature. 2014; 511:366–70.
Article18). Rao ALN. Genome packaging by spherical plant RNA viruses. Annu Rev Phytopathol. 2006; 44:61–87.
Article19). Schirawski J, Voyatzakis A, Zaccomer B, Bernardi F, Haenni AL. Identification and functional analysis of the turnip yellow mosaic tymovirus subgenomic promoter. J Virol. 2000; 74:11073–80.
Article20). Kim H-B, Chae K-H, Cho T-J. Genome size constraint in replication and packaging of Turnip yellow mosaic virus. J Bacteriol Virol. 2014; 44:188–96.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Sequence in Coat Protein Open Reading Frame Is Required for Turnip Yellow Mosaic Virus Replication
- Replication of Recombinant Flock House Virus RNA Encapsidated by Turnip Yellow Mosaic Virus Coat Proteins in Nicotiana benthamiana
- Genome Size Constraint in Replication and Packaging of Turnip Yellow Mosaic Virus
- Characterization of a Replication Element in the Coat Protein ORF of Turnip Yellow Mosaic Virus
- Read-through Mutation in the Coat Protein ORF Suppresses Turnip Yellow Mosaic Virus Subgenomic RNA Accumulation