Immune Netw.
2015 Apr;15(2):100-109. 10.4110/in.2015.15.2.100.
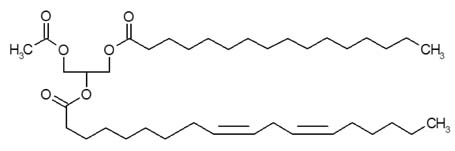
1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (EC-18) Modulates Th2 Immunity through Attenuation of IL-4 Expression
- Affiliations
-
- 1ENZYCHEM Lifesciences, Daejeon 305-732, Korea.
- 2Biomedical Translational Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon 305-806, Korea. wjkim@kribb.re.kr
- 3Soonchunhyang Medical Science Research Institute, College of Medicine Soonchunhyang University, Cheonan 330-721, Korea.
- KMID: 2168035
- DOI: http://doi.org/10.4110/in.2015.15.2.100
Abstract
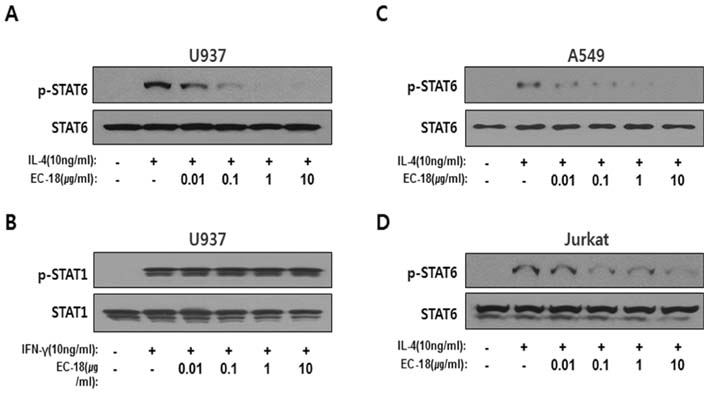
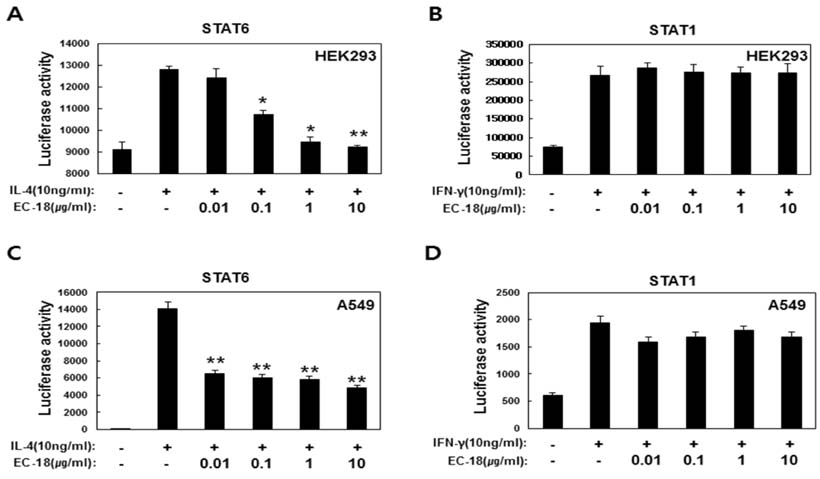
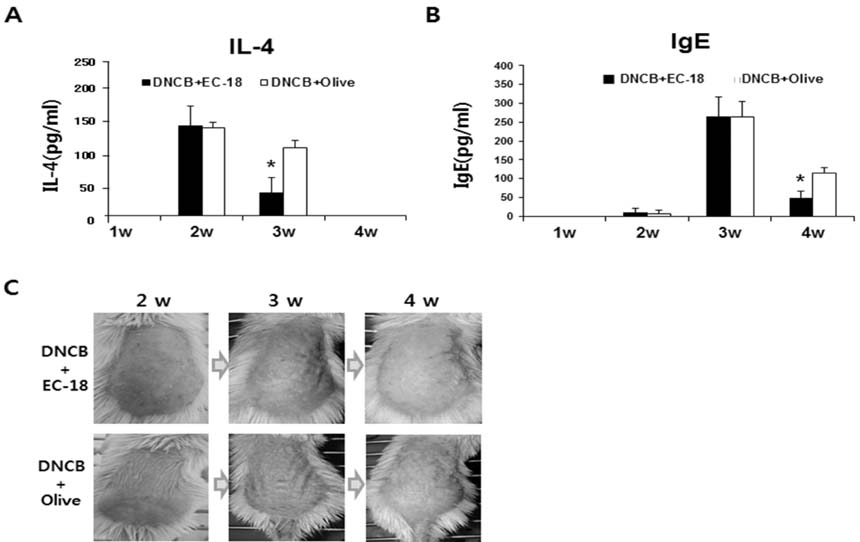
- Controlling balance between T-helper type 1 (Th1) and T-helper type 2 (Th2) plays a pivotal role in maintaining the biological rhythm of Th1/Th2 and circumventing diseases caused by Th1/Th2 imbalance. Interleukin 4 (IL-4) is a Th2-type cytokine and often associated with hypersensitivity-related diseases such as atopic dermatitis and allergies when overexpressed. In this study, we have tried to elucidate the function of 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (EC-18) as an essential modulator of Th1/Th2 balance. EC-18 has showed an inhibitory effect on the production of IL-4 in a dose-dependent manner. RT-PCR analysis has proved EC-18 affect the transcription of IL-4. By analyzing the phosphorylation status of Signal transducer and activator of transcription 6 (STAT6), which is a transcriptional activator of IL-4 expression, we discovered that EC-18 induced the decrease of STAT6 activity in several stimulated cell lines, which was also showed in STAT6 reporter analysis. Co-treatment of EC-18 significantly weakened atopy-like phenotypes in mice treated with an allergen. Collectively, our results suggest that EC-18 is a potent Th2 modulating factor by regulating the transcription of IL-4 via STAT6 modulation, and could be developed for immune-modulatory therapeutics.
Keyword
MeSH Terms
Figure
Reference
-
1. Kleiman R, Miller RW, Earle FR, Wolff IA. Optically active aceto-triglycerides of oil from Euonymus verrucosus seed. Lipids. 1966; 1:286–287.
Article2. Myher JJ, Kuksis A, Marai L, Sandra P. Identification of the more complex triacylglycerols in bovine milk fat by gas chromatography-mass spectrometry using polar capillary columns. J Chromatogr. 1988; 452:93–118.
Article3. Limb JK, Kim YH, Han SY, Jhon GJ. Isolation and characterization of monoacetyldiglycerides from bovine udder. J Lipid Res. 1999; 40:2169–2176.
Article4. Yang HO, Kim SH, Cho SH, Kim MG, Seo JY, Park JS, Jhon GJ, Han SY. Purification and structural determination of hematopoietic stem cell-stimulating monoacetyldiglycerides from Cervus nippon (deer antler). Chem Pharm Bull (Tokyo). 2004; 52:874–878.
Article5. Lee TS, Yook JS, Lee JS, Yoo CH, Lee JC, Lee CM, Lee WH. Preparation of glycerol derivatives and intermediates therefor. KR 10-0789323. 2005.6. Lee TS, Yook JS, Lee JS, Yoo CH, Lee JC, Lee CM, Lee WH. Preparation method of 1-palmitoyl-3-acetylglycerol, and preparation method of 1-palmitoyl-2-linoleoyl-3-acetylglycerol using same. WO 2013/043009 A2. 2013.7. Yang HO, Park JS, Cho SH, Yoon JY, Kim MG, Jhon GJ, Han SY, Kim SH. Stimulatory effects of monoacetyldiglycerides on hematopoiesis. Biol Pharm Bull. 2004; 27:1121–1125.
Article8. Kim MH, Chang HM, Kim TW, Lee SK, Park JS, Kim YH, Lee TY, Jang SJ, Suh CW, Lee TS, Kim SH, Lee SG. EC-18, a synthetic monoacetyldiacylglyceride, inhibits hematogenous metastasis of KIGB-5 biliary cancer cell in hamster model. J Korean Med Sci. 2009; 24:474–480.
Article9. Hong JJ, Koh Y, Park JS, Jung HD, Kim SH, Lee TS, Badellino MM. Enteral administration of a synthetic monoacetyldiglyceride improves survival in a murine model of abdominal sepsis. J Trauma. 2010; 68:62–68.
Article10. Shin IS, Shin NR, Jeon CM, Kwon OK, Sohn KY, Lee TS, Kim JW, Ahn KS, Oh SR. EC-18, a synthetic monoacetyldiglyceride (1-palmitoyl-2-linoleoyl-3-acetylglycerol), attenuates the asthmatic response in an aluminum hydroxide/ovalbumin-induced model of asthma1. Int Immunopharmacol. 2014; 18:116–123.
Article11. Coffman RL. Converging discoveries: the first reports of IL-4. J Immunol. 2013; 190:847–848.
Article12. Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013; 31:317–343.
Article13. Sehra S, Yao Y, Howell MD, Nguyen ET, Kansas GS, Leung DY, Travers JB, Kaplan MH. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010; 184:3186–3190.
Article14. Hatano Y, Adachi Y, Elias PM, Crumrine D, Sakai T, Kurahashi R, Katagiri K, Fujiwara S. The Th2 cytokine, interleukin-4, abrogates the cohesion of normal stratum corneum in mice: implications for pathogenesis of atopic dermatitis. Exp Dermatol. 2013; 22:30–35.
Article15. Bao L, Shi VY, Chan LS. IL-4 regulates chemokine CCL26 in keratinocytes through the Jak1, 2/Stat6 signal transduction pathway: Implication for atopic dermatitis. Mol Immunol. 2012; 50:91–97.
Article16. Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol. 2011; 2.
Article17. Zhu N, Gong Y, Chen XD, Zhang J, Long F, He J, Xia JW, Dong L. Association between the polymorphisms of interleukin-4, the interleukin-4 receptor gene and asthma. Chin Med J (Engl). 2013; 126:2943–2951.18. Nie W, Zang Y, Chen J, Xiu Q. Association between interleukin-4 receptor alpha chain (IL4RA) I50V and Q551R polymorphisms and asthma risk: an update meta-analysis. PLoS One. 2013; 8:e69120.19. Schuijs MJ, Willart MA, Hammad H, Lambrecht BN. Cytokine targets in airway inflammation. Curr Opin Pharmacol. 2013; 13:351–361.
Article20. Egawa M, Mukai K, Yoshikawa S, Iki M, Mukaida N, Kawano Y, Minegishi Y, Karasuyama H. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity. 2013; 38:570–580.
Article21. Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, Chatila TA, Oettgen HC. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol. 2013; 6:740–750.
Article22. Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996; 4:313–319.
Article23. Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007; 96:41–101.24. Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009; 31:539–550.
Article25. Wick KR, Berton MT. IL-4 induces serine phosphorylation of the STAT6 transactivation domain in B lymphocytes. Mol Immunol. 2000; 37:641–652.
Article26. Wu F, Li H, Jin L, Li X, Ma Y, You J, Li S, Xu Y. Deer antler base as a traditional Chinese medicine: a review of its traditional uses, chemistry and pharmacology. J Ethnopharmacol. 2013; 145:403–415.
Article27. Gilbey A, Perezgonzalez JD. Health benefits of deer and elk velvet antler supplements: a systematic review of randomised controlled studies. N Z Med J. 2012; 125:80–86.28. Mushaben EM, Kramer EL, Brandt EB, Khurana Hershey GK, Le Cras TD. Rapamycin attenuates airway hyperreactivity, goblet cells, and IgE in experimental allergic asthma. J Immunol. 2011; 187:5756–5763.
Article29. Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol. 2003; 3:721–732.
Article30. Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol. 2004; 4:978–988.
Article31. Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004; 20:429–440.
Article32. Liew FY. T(H)1 and T(H)2 cells: a historical perspective. Nat Rev Immunol. 2002; 2:55–60.
Article33. Yang XO, Zhang H, Kim BS, Niu X, Peng J, Chen Y, Kerketta R, Lee YH, Chang SH, Corry DB, Wang D, Watowich SS, Dong C. The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat Immunol. 2013; 14:732–740.
Article34. Knosp CA, Carroll HP, Elliott J, Saunders SP, Nel HJ, Amu S, Pratt JC, Spence S, Doran E, Cooke N, Jackson R, Swift J, Fitzgerald DC, Heaney LG, Fallon PG, Kissenpfennig A, Johnston JA. SOCS2 regulates T helper type 2 differentiation and the generation of type 2 allergic responses. J Exp Med. 2011; 208:1523–1531.
Article35. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010; 11:889–896.
Article36. Coffman RL. Origins of the T(H)1-T(H)2 model: a personal perspective. Nat Immunol. 2006; 7:539–541.
Article37. Sykes A, Edwards MR, Macintyre J, del Rosario A, Bakhsoliani E, Trujillo-Torralbo MB, Kon OM, Mallia P, McHale M, Johnston SL. Rhinovirus 16-induced IFN-alpha and IFN-beta are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012; 129:1506–1514.38. Baraldo S, Contoli M, Bazzan E, Turato G, Padovani A, Marku B, Calabrese F, Caramori G, Ballarin A, Snijders D, Barbato A, Saetta M, Papi A. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol. 2012; 130:1307–1314.
Article39. Huber JP, Ramos HJ, Gill MA, Farrar JD. Cutting edge: Type I IFN reverses human Th2 commitment and stability by suppressing GATA3. J Immunol. 2010; 185:813–817.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol on Immune Functions in Healthy Adults in a Randomized Controlled Trial
- IL-18Ralpha Mediated GATA-3 Induction by Th2 Cells: IL-12 Supports IL-18Ralpha Expression in Th2 Cells
- IL-4 Derived from Non-T Cells Induces Basophil- and IL-3-independent Th2 Immune Responses
- Regulation of Th2 Cell Immunity by Dendritic Cells
- N-acetyl-L-Cysteine Inhibits Functional Activation of Mouse Bone Marrow-Derived Dendritic Cells