Immune Netw.
2013 Dec;13(6):249-256. 10.4110/in.2013.13.6.249.
IL-4 Derived from Non-T Cells Induces Basophil- and IL-3-independent Th2 Immune Responses
- Affiliations
-
- 1Department of Immunology, Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, OH 44195, USA. minb@ccf.org
- 2Department of Immune Regulation and JST, CREST, Tokyo Medical and Dental University Graduate School, Tokyo, Japan.
- 3Division of Human Immunology, Center for Cancer Biology, Adelaide, South Australia, Australia.
- 4Genentech Inc., South San Francisco, CA 94080, USA.
- 5Malaghan Institute of Medical Research, Wellington, New Zealand.
- KMID: 2150785
- DOI: http://doi.org/10.4110/in.2013.13.6.249
Abstract
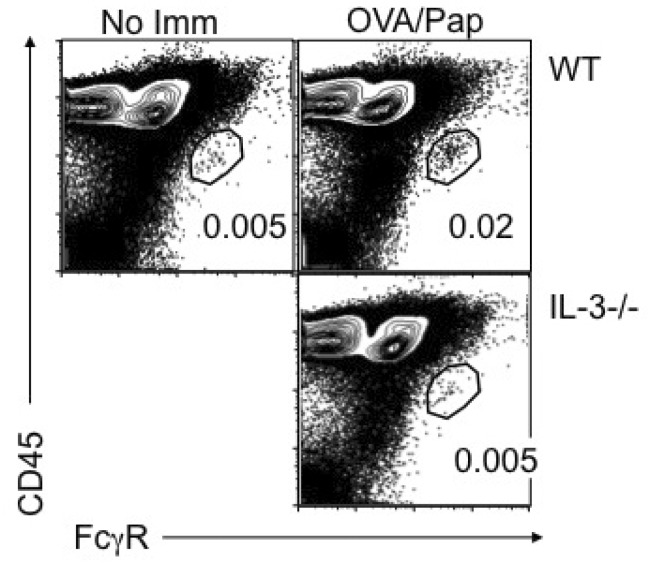
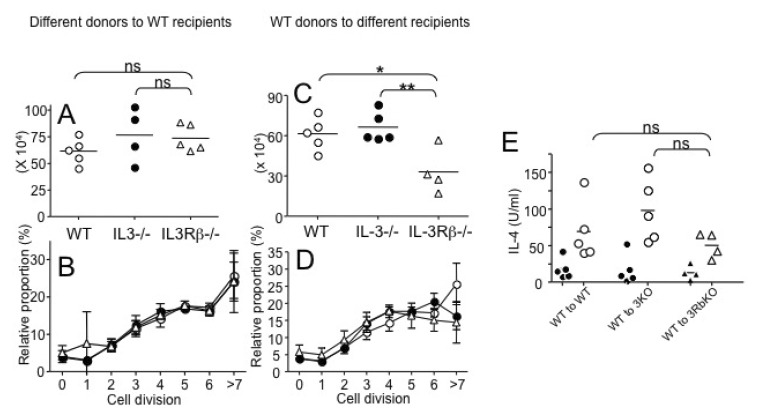
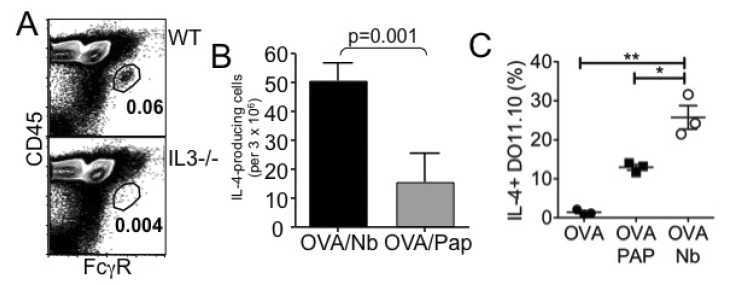
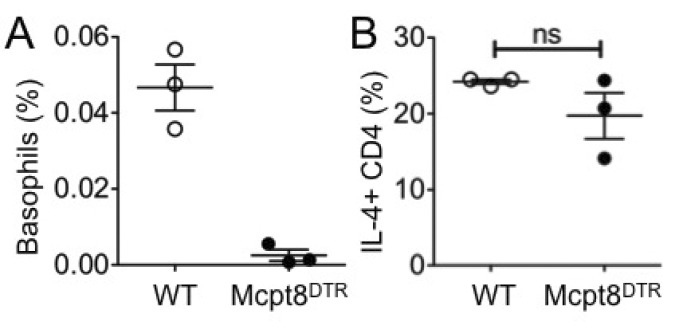
- How Th2 immunity develops in vivo remains obscure. Basophils have been considered key innate cells producing IL-4, a cytokine essential for Th2 immunity. Increasing evidence suggests that basophils are dispensable for the initiation of Th2 immunity. In this study, we revisited the role of basophils in Th2 immune responses induced by various types of adjuvants. Mice deficient in IL-3 or IL-3 receptor, in which basophil lymph node recruitment is completely abolished, fully developed wild type level Th2 CD4 T cell responses in response to parasite antigen or papain immunization. Similar finding was also observed in mice where basophils are inducibly ablated. Interestingly, IL-4-derived from non-T cells appeared to be critical for the generation of IL-4-producing CD4 T cells. Other Th2 promoting factors including IL-25 and thymic stromal lymphopoietin (TSLP) were dispensable. Therefore, our results suggest that IL-3- and basophil-independent in vivo Th2 immunity develops with the help of non-T cell-derived IL-4, offering an additional mechanism by which Th2 type immune responses arise in vivo.
Keyword
MeSH Terms
Figure
Reference
-
1. Karasuyama H, Mukai K, Obata K, Tsujimura Y, Wada T. Nonredundant roles of basophils in immunity. Annu Rev Immunol. 2011; 29:45–69. PMID: 21166539.
Article2. Min B. Basophils: what they 'can do' versus what they 'actually do'. Nat Immunol. 2008; 9:1333–1339. PMID: 19008933.
Article3. Sokol CL, Medzhitov R. Role of basophils in the initiation of Th2 responses. Curr Opin Immunol. 2010; 22:73–77. PMID: 20144855.
Article4. Siracusa MC, Perrigoue JG, Comeau MR, Artis D. New paradigms in basophil development, regulation and function. Immunol Cell Biol. 2010; 88:275–284. PMID: 20125116.
Article5. Nakanishi K. Basophils as APC in Th2 response in allergic inflammation and parasite infection. Curr Opin Immunol. 2010; 22:814–820. PMID: 21095110.
Article6. Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, Locksley RM. Genetic analysis of basophil function in vivo. Nat Immunol. 2011; 12:527–535. PMID: 21552267.7. Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010; 207:2097–2111. PMID: 20819925.
Article8. Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 2010; 184:1143–1147. PMID: 20038645.
Article9. Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, Hochweller K, Anderton SM, Hämmerling GJ, Maizels RM, MacDonald S. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010; 207:2089–2096. PMID: 20819926.10. Koyasu S, Moro K. Type 2 innate immune responses and the natural helper cell. Immunology. 2011; 132:475–481. PMID: 21323663.
Article11. Barlow JL, McKenzie AN. IL-25: a key requirement for the regulation of type-2 immunity. Biofactors. 2009; 35:178–182. PMID: 19449446.
Article12. Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010; 31:407–413. PMID: 20951092.
Article13. Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010; 464:1362–1366. PMID: 20200520.
Article14. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010; 464:1367–1370. PMID: 20200518.
Article15. Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007; 204:1837–1847. PMID: 17635955.
Article16. Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, Dudek EC, Kubo M, Cianferoni A, Spergel JM, Ziegler SF, Comeau MR, Artis D. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011; 477:229–233. PMID: 21841801.
Article17. Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, Hirokawa K, Etori M, Yamashita M, Kubota T, Minegishi Y, Yonekawa H, Karasuyama H. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005; 23:191–202. PMID: 16111637.
Article18. Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, Obata K, Ishikawa R, Yoshikawa S, Mukai K, Kawano Y, Minegishi Y, Yokozeki H, Watanabe N, Karasuyama H. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010; 120:2867–2875. PMID: 20664169.
Article19. Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010; 184:344–350. PMID: 19955520.
Article20. Asquith KL, Ramshaw HS, Hansbro PM, Beagley KW, Lopez AF, Foster PS. The IL-3/IL-5/GM-CSF common receptor plays a pivotal role in the regulation of Th2 immunity and allergic airway inflammation. J Immunol. 2008; 180:1199–1206. PMID: 18178860.21. Hu-Li J, Pannetier C, Guo L, Löhning M, Gu H, Watson C, Assenmacher M, Radbruch A, Paul WE. Regulation of expression of IL-4 alleles: analysis using a chimeric GFP/IL-4 gene. Immunity. 2001; 14:1–11. PMID: 11163225.22. Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998; 392:90–93. PMID: 9510253.
Article23. Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008; 9:310–318. PMID: 18300366.
Article24. van Panhuys N, Forbes E, van Panhuys N, Min B, Le Gros G. Basophils are the major producers of IL-4 during primary helminth infection. J Immunol. 2011; 186:2719–2728. PMID: 21270410.
Article25. Noben-Trauth N, Hu-Li J, Paul WE. Conventional, naive CD4+ T cells provide an initial source of IL-4 during Th2 differentiation. J Immunol. 2000; 165:3620–3625. PMID: 11034364.26. Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, Tokuhisa T, Iwamoto I, Nakajima H. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006; 118:606–614. PMID: 16950278.
Article27. Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009; 10:697–705. PMID: 19465906.28. Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009; 10:706–712. PMID: 19465908.29. Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006; 203:1105–1116. PMID: 16606668.
Article30. Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009; 39:798–806. PMID: 19400908.
Article31. Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy. 2010; 40:200–208. PMID: 19906013.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of Th2 Cell Immunity by Dendritic Cells
- IL-18Ralpha Mediated GATA-3 Induction by Th2 Cells: IL-12 Supports IL-18Ralpha Expression in Th2 Cells
- Panonychus citri Can Induce T-helper Type 2 Immune Responses via the Release of Thymic Stromal Lymphopoietin and IL-4
- Regulation and Function of Th17 Cells
- Role of IL-23 and Th17 Cells in Airway Inflammation in Asthma