Immune Netw.
2015 Apr;15(2):73-82. 10.4110/in.2015.15.2.73.
Caspase-1 Independent Viral Clearance and Adaptive Immunity Against Mucosal Respiratory Syncytial Virus Infection
- Affiliations
-
- 1Laboratory of Host Defenses, Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon 305-338, Korea. heungkyu.lee@kaist.ac.kr
- KMID: 2168032
- DOI: http://doi.org/10.4110/in.2015.15.2.73
Abstract
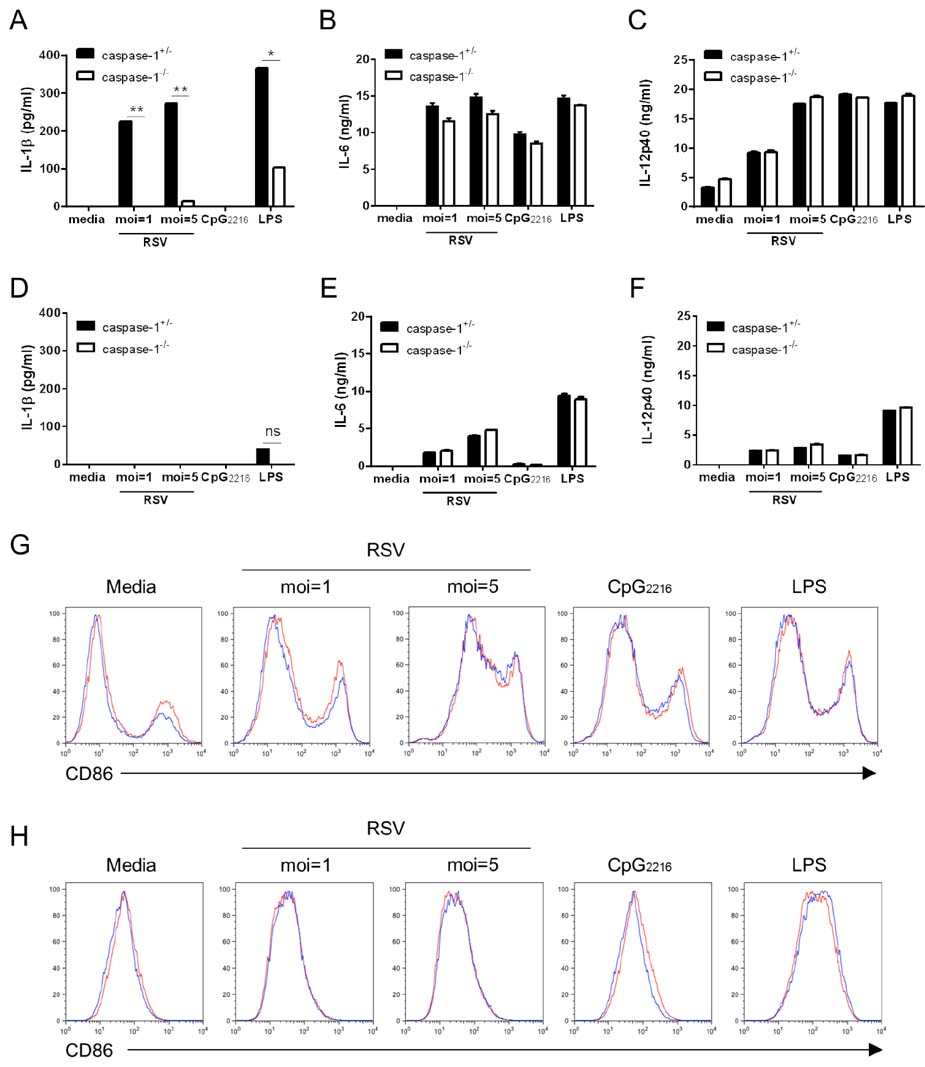
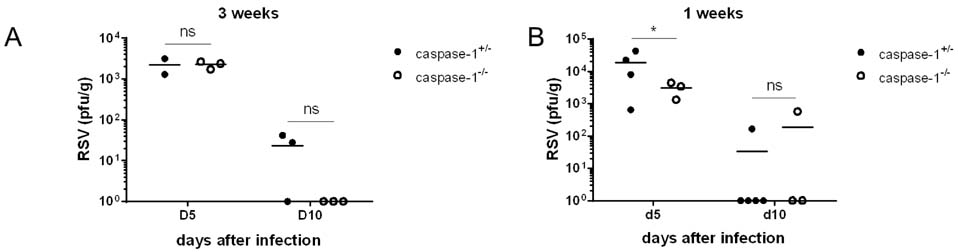
- Respiratory syncytial virus (RSV) infection is recognized by the innate immune system through Toll like receptors (TLRs) and retinoic acid inducible gene I. These pathways lead to the activation of type I interferons and resistance to infection. In contrast to TLRs, very few studies have examined the role of NOD-like receptors in viral recognition and induction of adaptive immune responses to RSV. Caspase-1 plays an essential role in the immune response via the maturation of the proinflammatory cytokines IL-1beta and IL-18. However, the role of caspase-1 in RSV infection in vivo is unknown. We demonstrate that RSV infection induces IL-1beta secretion and that caspase-1 deficiency in bone marrow derived dendritic cells leads to defective IL-1beta production, while normal RSV viral clearance and T cell responses are observed in caspase-1 deficient mice following respiratory infection with RSV. The frequencies of IFN-gamma producing or RSV specific T cells in lungs from caspase-1 deficient mice are not impaired. In addition, we demonstrate that caspase-1 deficient neonatal or young mice also exhibit normal immune responses. Furthermore, we find that IL-1R deficient mice infected with RSV exhibit normal Th1 and cytotoxic T lymphocytes (CTL) immune responses. Collectively, these results demonstrate that in contrast to TLR pathways, caspase-1 might not play a central role in the induction of Th1 and CTL immune responses to RSV.
Keyword
MeSH Terms
Figure
Reference
-
1. Collins PL. The molecular biology of human respiratory syncytial virus (RSV) of the genus Pneumovirus. New York: Springer. Plenum Press;1991. p. 103–162.2. Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008; 82:2040–2055.
Article3. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005; 352:1749–1759.
Article4. Sollberger G, Strittmatter GE, Garstkiewicz M, Sand J, Beer HD. Caspase-1: the inflammasome and beyond. Innate Immun. 2014; 20:115–125.
Article5. Case CL. Regulating caspase-1 during infection: roles of NLRs, AIM2, and ASC. Yale J Biol Med. 2011; 84:333–343.6. Kim TH, Lee HK. Differential roles of lung dendritic cell subsets against respiratory virus infection. Immune Netw. 2014; 14:128–137.
Article7. Kim TH, Lee HK. Innate immune recognition of respiratory syncytial virus infection. BMB Rrep. 2014; 47:184–191.
Article8. Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009; 10:241–247.
Article9. Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008; 223:20–38.
Article10. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009; 27:519–550.
Article11. Allen IC, Scull MA, Moore CB, Holl EK, Elvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009; 30:556–565.
Article12. Thomas PG, Dash P, Aldridge JR Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009; 30:566–575.
Article13. Fang R, Tsuchiya K, Kawamura I, Shen Y, Hara H, Sakai S, Yamamoto T, Fernandes-Alnemri T, Yang R, Hernandez-Cuellar E, Dewamitta SR, Xu Y, Qu H, Alnemri ES, Mitsuyama M. Critical roles of ASC inflammasomes in caspase-1 activation and host innate resistance to Streptococcus pneumoniae infection. J Immunol. 2011; 187:4890–4899.
Article14. Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010; 11:1136–1142.
Article15. Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009; 206:79–87.
Article16. Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010; 11:404–410.
Article17. Takeuchi R, Tsutsumi H, Osaki M, Haseyama K, Mizue N, Chiba S. Respiratory syncytial virus infection of human alveolar epithelial cells enhances interferon regulatory factor 1 and interleukin-1beta-converting enzyme gene expression but does not cause apoptosis. J Virol. 1998; 72:4498–4502.
Article18. Takeuchi R, Tsutsumi H, Osaki M, Sone S, Imai S, Chiba S. Respiratory syncytial virus infection of neonatal monocytes stimulates synthesis of interferon regulatory factor 1 and interleukin-1beta (IL-1beta)-converting enzyme and secretion of IL-1beta. J Virol. 1998; 72:837–840.
Article19. Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, Morris IR, Allen IC, Ting JP, Bose S. TLR2/MyD88/NF-kappaB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One. 2012; 7:e29695.20. Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995; 267:2000–2003.
Article21. Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997; 159:3364–3371.22. Kim S, Joo DH, Lee JB, Shim BS, Cheon IS, Jang JE, Song HH, Kim KH, Song MK, Chang J. Dual role of respiratory syncytial virus glycoprotein fragment as a mucosal immunogen and chemotactic adjuvant. PLoS One. 2012; 7:e32226.
Article23. Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996; 87:2095–2147.
Article24. Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010; 3:cm1.
Article25. Pang IK, Iwasaki A. Inflammasomes as mediators of immunity against influenza virus. Trends Immunol. 2011; 32:34–41.
Article26. Taylor G, Stott EJ, Hughes M, Collins AP. Respiratory syncytial virus infection in mice. Infect Immun. 1984; 43:649–655.
Article27. Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991; 88:1026–1033.
Article28. Ostler T, Davidson W, Ehl S. Virus clearance and immunopathology by CD8+ T cells during infection with respiratory syncytial virus are mediated by IFN-γ. Eur J Immunol. 2002; 32:2117–2123.
Article29. Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998; 8:383–390.
Article30. Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997; 386:619–623.
Article31. Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004; 4:553–564.
Article32. Cormier SA, You D, Honnegowda S. The use of a neonatal mouse model to study respiratory syncytial virus infections. Expert Rev Anti Infect Ther. 2010; 8:1371–1380.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Hot Pursuit of the First Vaccine Against Respiratory Syncytial Virus
- Differential Roles of Lung Dendritic Cell Subsets Against Respiratory Virus Infection
- Prophylactic and Therapeutic Modulation of Innate and Adaptive Immunity Against Mucosal Infection of Herpes Simplex Virus
- Nosocomial respiratory syncytial virus infection in a newborn nursery
- Respiratory Syncytial Virus (RSV) Modulation at the Virus-Host Interface Affects Immune Outcome and Disease Pathogenesis