Immune Netw.
2014 Apr;14(2):100-106. 10.4110/in.2014.14.2.100.
Shigella flexneri Inhibits Intestinal Inflammation by Modulation of Host Sphingosine-1-Phosphate in Mice
- Affiliations
-
- 1Laboratory of Microbiology, College of Pharmacy, Ajou University, Suwon 443-749, Korea. sychang@ajou.ac.kr
- 2Mucosal Immunology Laboratory, Department of Convergence Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul 138-736, Korea. mnkweon@amc.seoul.kr
- 3Laboratory of Microbiology and Immunology, College of Pharmacy, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 2168020
- DOI: http://doi.org/10.4110/in.2014.14.2.100
Abstract
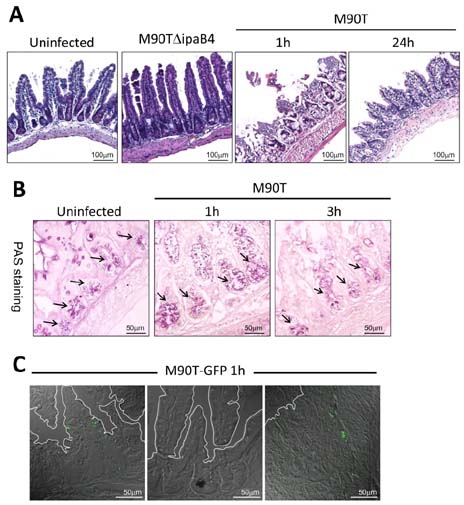
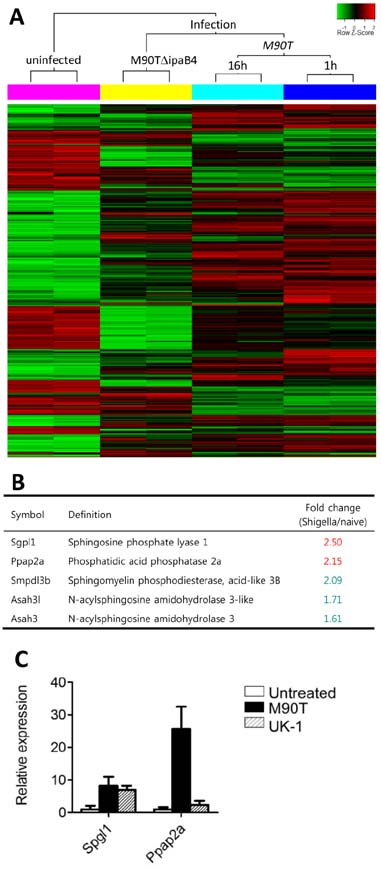
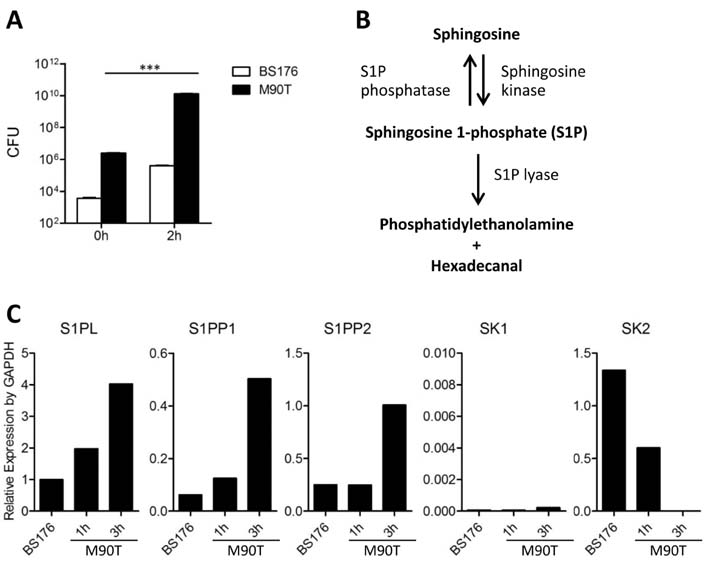
- Infection with invasive Shigella species results in intestinal inflammation in humans but no symptoms in adult mice. To investigate why adult mice are resistant to invasive shigellae, 6~8-week-old mice were infected orally with S. flexneri 5a. Shigellae successfully colonized the small and large intestines. Mild cell death was seen but no inflammation. The infected bacteria were cleared 24 hours later. Microarray analysis of infected intestinal tissue showed that several genes that are involved with the sphingosine-1-phosphate (S1P) signaling pathway, a lipid mediator which mediates immune responses, were altered significantly. Shigella infection of a human intestinal cell line modulated host S1P-related genes to reduce S1P levels. In addition, co-administration of S1P with shigellae could induce inflammatory responses in the gut. Here we propose that Shigella species have evasion mechanisms that dampen host inflammatory responses by lowering host S1P levels in the gut of adult mice.
MeSH Terms
Figure
Reference
-
1. Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999; 77:651–666.2. Ogawa M, Handa Y, Ashida H, Suzuki M, Sasakawa C. The versatility of Shigella effectors. Nat Rev Microbiol. 2008; 6:11–16.
Article3. Kweon MN. Shigellosis: the current status of vaccine development. Curr Opin Infect Dis. 2008; 21:313–318.
Article4. Ashida H, Ogawa M, Mimuro H, Kobayashi T, Sanada T, Sasakawa C. Shigella are versatile mucosal pathogens that circumvent the host innate immune system. Curr Opin Immunol. 2011; 23:448–455.
Article5. Shim DH, Suzuki T, Chang SY, Park SM, Sansonetti PJ, Sasakawa C, Kweon MN. New animal model of shigellosis in the Guinea pig: its usefulness for protective efficacy studies. J Immunol. 2007; 178:2476–2482.
Article6. Fernandez MI, Thuizat A, Pedron T, Neutra M, Phalipon A, Sansonetti PJ. A newborn mouse model for the study of intestinal pathogenesis of shigellosis. Cell Microbiol. 2003; 5:481–491.
Article7. Shim DH, Ryu S, Kweon MN. Defensins play a crucial role in protecting mice against oral Shigella flexneri infection. Biochem Biophys Res Commun. 2010; 401:554–560.
Article8. Ashida H, Mimuro H, Ogawa M, Kobayashi T, Sanada T, Kim M, Sasakawa C. Cell death and infection: a double-edged sword for host and pathogen survival. J Cell Biol. 2011; 195:931–942.
Article9. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009; 7:99–109.
Article10. Sanada T, Kim M, Mimuro H, Suzuki M, Ogawa M, Oyama A, Ashida H, Kobayashi T, Koyama T, Nagai S, Shibata Y, Gohda J, Inoue J, Mizushima T, Sasakawa C. The Shigella flexneri effector OspI deamidates UBC13 to dampen the inflammatory response. Nature. 2012; 483:623–626.
Article11. Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. Escape of intracellular Shigella from autophagy. Science. 2005; 307:727–731.12. Ogawa M, Yoshikawa Y, Kobayashi T, Mimuro H, Fukumatsu M, Kiga K, Piao Z, Ashida H, Yoshida M, Kakuta S, Koyama T, Goto Y, Nagatake T, Nagai S, Kiyono H, Kawalec M, Reichhart JM, Sasakawa C. A Tecpr1-dependent selective autophagy pathway targets bacterial pathogens. Cell Host Microbe. 2011; 9:376–389.
Article13. Chang SY, Lee SN, Yang JY, Kim DW, Yoon JH, Ko HJ, Ogawa M, Sasakawa C, Kweon MN. Autophagy controls an intrinsic host defense to bacteria by promoting epithelial cell survival: a murine model. PLoS One. 2013; 8:e81095.
Article14. Yang JY, Lee SN, Chang SY, Ko HJ, Ryu S, Kweon MN. A mouse model of shigellosis by intraperitoneal infection. J Infect Dis. 2014; 209:203–215.
Article15. Le Stunff H, Mikami A, Giussani P, Hobson JP, Jolly PS, Milstien S, Spiegel S. Role of sphingosine-1-phosphate phosphatase 1 in epidermal growth factor-induced chemotaxis. J Biol Chem. 2004; 279:34290–34297.
Article16. Ashida H, Ogawa M, Kim M, Mimuro H, Sasakawa C. Bacteria and host interactions in the gut epithelial barrier. Nat Chem Biol. 2011; 8:36–45.
Article17. Sansonetti PJ. Shigellosis: an old disease in new clothes? PLoS Med. 2006; 3:e354.
Article18. Le Stunff H, Milstien S, Spiegel S. Generation and metabolism of bioactive sphingosine-1-phosphate. J Cell Biochem. 2004; 92:882–899.
Article19. Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011; 11:403–415.
Article20. Chi H. Sphingosine-1-phosphate and immune regulation: trafficking and beyond. Trends Pharmacol Sci. 2011; 32:16–24.
Article21. Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010; 465:1084–1088.
Article22. Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011; 146:980–991.
Article23. Ashida H, Ogawa M, Mimuro H, Sasakawa C. Shigella infection of intestinal epithelium and circumvention of the host innate defense system. Curr Top Microbiol Immunol. 2009; 337:231–255.
Article24. Tran Van Nhieu G, Bourdet-Sicard R, Dumenil G, Blocker A, Sansonetti PJ. Bacterial signals and cell responses during Shigella entry into epithelial cells. Cell Microbiol. 2000; 2:187–193.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Infection of Extended-Spectrum β-Lactamase Producing Shigella flexneri in Children Attending a Childcare Center in Korea
- Deficiency of Sphingosine-1-Phosphate Receptor 2 (S1Pâ‚‚) Attenuates Bleomycin-Induced Pulmonary Fibrosis
- Shigella flexneri bacteremia: A case report
- Epidemiological Characterization of Shigella flexneri Isolates in Korea and the Analysis of Pulsed - Field Gel Electrophoresis Patterns
- Shigella flexneri infection in a newly acquired rhesus macaque (Macaca mulatta)