Allergy Asthma Immunol Res.
2013 Jan;5(1):48-54. 10.4168/aair.2013.5.1.48.
In Vitro Induction of Allergen-Specific Interleukin-10-Producing Regulatory B Cell Responses by Interferon-gamma in Non-Immunoglobulin E-Mediated Milk Allergy
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Eulji University, Daejeon, Korea.
- 2Department of Pediatrics, Subdivision of Allergy and Clinical Immunology, Chungnam National University Hospital, Daejeon, Korea. immlee@cnu.ac.kr
- 3Department of Pediatrics, School of Medicine, Chungnam National University, Daejeon, Korea.
- KMID: 2167006
- DOI: http://doi.org/10.4168/aair.2013.5.1.48
Abstract
- PURPOSE
Specific oral immunotherapy (SOIT) using interferon-gamma (IFN-gamma) has been successful as a food allergy treatment. Interleukin-10 (IL-10)-producing regulatory B cells (Br1s) play a role in immune tolerance to food allergens. In addition, IFN-gamma shows tolerogenic effects on allergen-induced Br1 responses.
METHODS
Eleven patients that were allergic to cow's milk and 12 milk-tolerant subjects were selected by double-blind placebo-controlled food challenge (DBPCFC) and clinical characteristics. The immunomodulatory effects of IFN-gamma on allergen-specific Br1 responses were evaluated in 6 milk allergy patients and 8 milk-tolerant subjects. Peripheral blood mononuclear cells (PBMCs) from subjects were stimulated with casein and/or IFN-gamma and analyzed by flow cytometry.
RESULTS
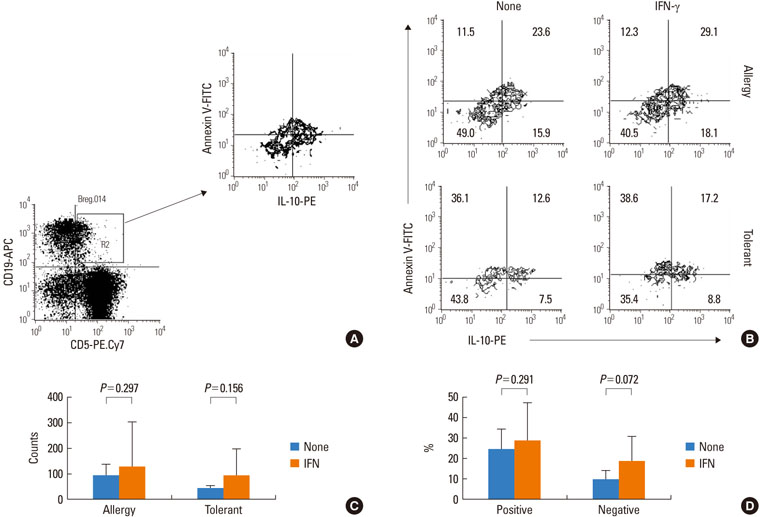
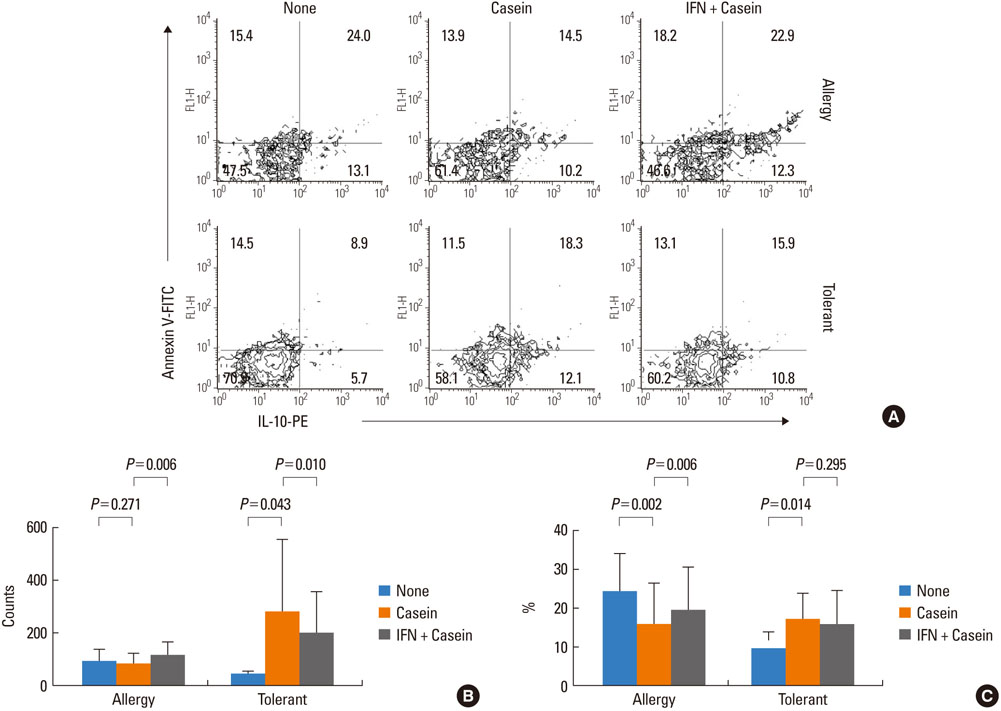
IFN-gamma had no effect on total cell counts or the proportion of Br1 cells in PBMCs. IFN-gamma stimulation did not change total Br1 cell counts or the percentage of Br1s among CD5(+) B cells in the milk allergy or the milk-tolerant groups. In the milk allergy group, Br1 counts were not different between the control and the casein stimulation but significantly increased in the IFN-gamma + casein stimulated cells, and the Br1 fractions were decreased after casein stimulation and recovered in the addition of IFN-gamma for stimulation. In the milk-tolerant group, Br1 counts increased in the casein stimulated cells and in the IFN-gamma + casein stimulated cells, but the increase was significantly less when IFN-gamma was added, and the Br1 fractions were increased after casein stimulation and IFN-gamma + casein stimulation, that was not significant when IFN-gamma was added.
CONCLUSIONS
IFN-gamma-induced allergen-specific Br1 responses in the PBMCs of milk allergy patients play a role in milk allergen-specific tolerance induction in vitro. Further investigations into the molecular immunological mechanisms underlying the induction of allergen-specific Br1 responses are needed.
Keyword
MeSH Terms
Figure
Reference
-
1. Werfel T, Ballmer-Weber B, Eigenmann PA, Niggemann B, Rancé F, Turjanmaa K, Worm M. Eczematous reactions to food in atopic eczema: position paper of the EAACI and GA2LEN. Allergy. 2007. 62:723–728.2. Wang J, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Allergy Asthma Immunol Res. 2009. 1:19–29.3. Cianferoni A, Spergel JM. Food allergy: review, classification and diagnosis. Allergol Int. 2009. 58:457–466.4. Fogg MI, Spergel JM. Management of food allergies. Expert Opin Pharmacother. 2003. 4:1025–1037.5. Wüthrich B. Oral desensitization with cow's milk in cow's milk allergy. Pro! Monogr Allergy. 1996. 32:236–240.6. Rolinck-Werninghaus C, Staden U, Mehl A, Hamelmann E, Beyer K, Niggemann B. Specific oral tolerance induction with food in children: transient or persistent effect on food allergy? Allergy. 2005. 60:1320–1322.7. Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007. 62:1261–1269.8. Noh G, Lee SS. Marone G, editor. Effects of IFN-γ on milk-specific oral tolerance induction for milk allergy in atopic dermatitis: food-specific oral tolerance induction using IFN-γ as adjuvant. Clinical immunology and allergy in medicine. 2003. Naples, Italy: JGC Editions;475–496.9. Grewe M, Bruijnzeel-Koomen CA, Schöpf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, Krutmann J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998. 19:359–361.10. Noh G, Lee SS. A pilot study of interferon-gamma-induced specific oral tolerance induction (ISOTI) for immunoglobulin E-mediated anaphylactic food allergy. J Interferon Cytokine Res. 2009. 29:667–675.11. Lee JH, Noh G, Noh J, Lee S, Choi WS, Kim HS, Lee K, Choi S, Jin H, Cho S, Lee S. Clinical characteristics of oral tolerance induction of IgE-mediated and non-IgE-mediated food allergy using interferon gamma. Allergy Asthma Proc. 2010. 31:e39–e47.12. Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009. 123:43–52.13. Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006. 176:705–710.14. Lee JH, Noh J, Noh G, Kim HS, Mun SH, Choi WS, Cho S, Lee S. Allergen-specific B cell subset responses in cow's milk allergy of late eczematous reactions in atopic dermatitis. Cell Immunol. 2010. 262:44–51.15. Noh J, Lee JH, Noh G, Bang SY, Kim HS, Choi WS, Cho S, Lee SS. Characterisation of allergen-specific responses of IL-10-producing regulatory B cells (Br1) in Cow Milk Allergy. Cell Immunol. 2010. 264:143–149.16. Noh G, Lee JH. Regulatory B cells and allergic diseases. Allergy Asthma Immunol Res. 2011. 3:168–177.17. Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010. 125:1114–1124.18. Noh J, Noh G, Lee SJ, Lee JH, Kim A, Kim HS, Choi WS. Tolerogenic effects of interferon-gamma with induction of allergen-specific interleukin-10-producing regulatory B cell (Br1) changes in non-IgE-mediated food allergy. Cell Immunol. 2012. 273:140–149.19. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980. 59:Suppl 92. 44–47.20. Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, Bush RK, Metcalfe DD. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol. 1988. 82:986–997.21. Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Adverse Reactions to Food Committee of American Academy of Allergy, Asthma & Immunology. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009. 123:S365–S383.22. Hauk PJ. The role of food allergy in atopic dermatitis. Curr Allergy Asthma Rep. 2008. 8:188–194.23. Werfel T, Breuer K. Role of food allergy in atopic dermatitis. Curr Opin Allergy Clin Immunol. 2004. 4:379–385.24. Sprikkelman AB, Tupker RA, Burgerhof H, Schouten JP, Brand PL, Heymans HS, van Aalderen WM. Severity scoring of atopic dermatitis: a comparison of three scoring systems. Allergy. 1997. 52:944–949.25. Szczepanik M, Akahira-Azuma M, Bryniarski K, Tsuji RF, Kawikova I, Ptak W, Kiener C, Campos RA, Askenase PW. B-1 B cells mediate required early T cell recruitment to elicit protein-induced delayed-type hypersensitivity. J Immunol. 2003. 171:6225–6235.26. Kawikova I, Paliwal V, Szczepanik M, Itakura A, Fukui M, Campos RA, Geba GP, Homer RJ, Iliopoulou BP, Pober JS, Tsuji RF, Askenase PW. Airway hyper-reactivity mediated by B-1 cell immunoglobulin M antibody generating complement C5a at 1 day post-immunization in a murine hapten model of non-atopic asthma. Immunology. 2004. 113:234–245.27. Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003. 111:1255–1261.28. Vacchio MS, Hodes RJ. CD28 costimulation is required for in vivo induction of peripheral tolerance in CD8 T cells. J Exp Med. 2003. 197:19–26.29. Koide N, Sugiyama T, Mori I, Mu MM, Hamano T, Yoshida T, Yokochi T. Change of mouse CD5(+) B1 cells to a macrophage-like morphology induced by gamma interferon and inhibited by interleukin-4. Clin Diagn Lab Immunol. 2002. 9:1169–1174.30. Sütas Y, Hurme M, Isolauri E. Oral cow milk challenge abolishes antigen-specific interferon-gamma production in the peripheral blood of children with atopic dermatitis and cow milk allergy. Clin Exp Allergy. 1997. 27:277–283.31. Lee HO, Miller SD, Hurst SD, Tan LJ, Cooper CJ, Barrett TA. Interferon gamma induction during oral tolerance reduces T-cell migration to sites of inflammation. Gastroenterology. 2000. 119:129–138.32. Suomalainen H, Soppi E, Laine S, Isolauri E. Immunologic disturbances in cow's milk allergy, 2: Evidence for defective interferon-gamma generation. Pediatr Allergy Immunol. 1993. 4:203–207.33. Qu C, Srivastava K, Ko J, Zhang TF, Sampson HA, Li XM. Induction of tolerance after establishment of peanut allergy by the food allergy herbal formula-2 is associated with up-regulation of interferon-gamma. Clin Exp Allergy. 2007. 37:846–855.34. Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009. 123:443–451.35. Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007. 120:707–713.36. Weiner HL. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001. 3:947–954.37. Sato MN, Fusaro AE, Victor JR, Oliveira CR, Futata ET, Maciel M, Carvalho AF, Duarte AJ. Oral tolerance induction in dermatophagoides pteronyssinus-sensitized mice induces inhibition of IgE response and upregulation of TGF-beta secretion. J Interferon Cytokine Res. 2001. 21:827–833.38. Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, Akdis CA. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003. 33:1205–1214.39. Kehrl JH, Taylor A, Kim SJ, Fauci AS. Transforming growth factor-beta is a potent negative regulator of human lymphocytes. Ann N Y Acad Sci. 1991. 628:345–353.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Update in the Mechanisms of Allergen-Specific Immunotheraphy
- The effects of interleukin-4 and interferon-gamma on the expression of FcepsilonR II on B cells
- The effect of house dust mite conventional immunotherapy on the production of IL-4 and interferon-gamma from the peripheral blood T cells in asthmatic children
- Regulatory B Cells and Allergic Diseases
- Mechanisms of immune tolerance to allergens in children