Ann Surg Treat Res.
2015 Sep;89(3):145-150. 10.4174/astr.2015.89.3.145.
De novo hepatitis B virus infection developing after liver transplantation using a graft positive for hepatitis B core antibody
- Affiliations
-
- 1Department of Surgery, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. kimdg@catholic.ac.kr
- 2Department of Internal Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2166945
- DOI: http://doi.org/10.4174/astr.2015.89.3.145
Abstract
- PURPOSE
The use of hepatitis B core antibody (HBcAb)-positive grafts is increasing, especially where hepatitis B is endemic. However, this remains controversial because of the risk of development of de novo HBV infection.
METHODS
We collected information obtained between January 2000 and December 2012 and retrospectively analyzed data on 187 HBsAg-negative donors and recipients were analyzed retrospectively. De novo HBV infection was defined as development of HBsAg positivity with or without detection of HBV DNA.
RESULTS
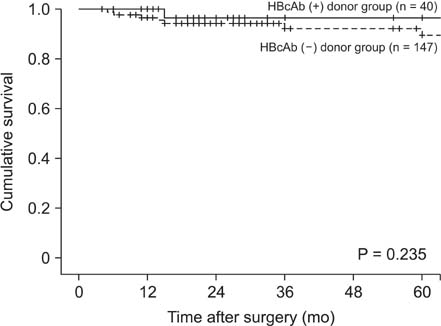
Forty patients (21.4%) received HBcAb-positive grafts. Survival rate did not differ by donor HBcAb status (P = 0.466). De novo HBV infection occurred in five patients (12.5%) who were not treated with anti-HBV prophylaxis, and was significantly more prevalent in hepatitis B surface antibody (HBsAb)- and HBcAb-negative than HBsAb- and HBcAb-positive recipients (50% vs. 4.2%, P = 0.049). All patients except one were treated with entecavir with/without antihepatitis B immunoglobulin and four were negative in terms of HBV DNA seroconversion. No patient died.
CONCLUSION
HBcAb-positive grafts are safe without survival difference. However, the risk of de novo hepatitis B virus infection was significantly increased in HBsAb- and HBcAb-negative recipients. All patients were successfully treated even after recurrence.
MeSH Terms
Figure
Reference
-
1. Montalti R, Nardo B, Bertelli R, Beltempo P, Puviani L, Vivarelli M, et al. Donor pool expansion in liver transplantation. Transplant Proc. 2004; 36:520–522.2. Prieto M, Gomez MD, Berenguer M, Cordoba J, Rayon JM, Pastor M, et al. De novo hepatitis B after liver transplantation from hepatitis B core antibody-positive donors in an area with high prevalence of anti-HBc positivity in the donor population. Liver Transpl. 2001; 7:51–58.3. Roche B, Samuel D, Gigou M, Feray C, Virot V, Schmets L, et al. De novo and apparent de novo hepatitis B virus infection after liver transplantation. J Hepatol. 1997; 26:517–526.4. Angelico M, Nardi A, Marianelli T, Caccamo L, Romagnoli R, Tisone G, et al. Hepatitis B-core antibody positive donors in liver transplantation and their impact on graft survival: evidence from the Liver Match cohort study. J Hepatol. 2013; 58:715–723.5. Celebi Kobak A, Karasu Z, Kilic M, Ozacar T, Tekin F, Gunsar F, et al. Living donor liver transplantation from hepatitis B core antibody positive donors. Transplant Proc. 2007; 39:1488–1490.6. Hwang S, Moon DB, Lee SG, Park KM, Kim KH, Ahn CS, et al. Safety of anti-hepatitis B core antibody-positive donors for living-donor liver transplantation. Transplantation. 2003; 75:3 Suppl. S45–S48.7. Chang MS, Olsen SK, Pichardo EM, Stiles JB, Rosenthal-Cogan L, Brubaker WD, et al. Prevention of de novo hepatitis B in recipients of core antibody-positive livers with lamivudine and other nucleos(t)ides: a 12-year experience. Transplantation. 2013; 95:960–965.8. Douglas DD, Rakela J, Wright TL, Krom RA, Wiesner RH. The clinical course of transplantation-associated de novo hepatitis B infection in the liver transplant recipient. Liver Transpl Surg. 1997; 3:105–111.9. Villamil I, Gonzalez-Quintela A, Aguilera A, Tome S, Otero E, Castroagudin FJ, et al. Truly de novo hepatitis B after liver transplantation. Am J Gastroenterol. 2004; 99:767–768.10. Brock GN, Mostajabi F, Ferguson N, Carrubba CJ, Eng M, Buell JF, et al. Prophylaxis against de novo hepatitis B for liver transplantation utilizing hep B core (+) donors: does hepatitis B immunoglobulin provide a survival advantage? Transpl Int. 2011; 24:570–581.11. Douglas DD, Rakela J, Taswell HF, Krom RA, Wiesner RH. Hepatitis B virus replication patterns after orthotopic liver transplantation: de novo versus recurrent infection. Transplant Proc. 1993; 25:1755–1757.12. Skagen CL, Jou JH, Said A. Risk of de novo hepatitis in liver recipients from hepatitis-B core antibody-positive grafts: a systematic analysis. Clin Transplant. 2011; 25:E243–E249.13. Dodson SF. Prevention of de novo hepatitis B infection after liver transplantation with allografts from hepatitis B core antibody positive donors. Clin Transplant. 2000; 14:Suppl 2. 20–24.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prophylaxis for Hepatitis B Core Antibody-Positive Donors after Liver Transplantation
- Adult Living Donor Liver Transplantation Using Hepatitis B Core Antibody-Positive Grafts in Korea, a Hepatitis B-endemic Region
- De novo hepatitis B virus infection after liver transplantation from anti-hepatitis B core antibody positive donor: a 20-year experience at a single center
- Detection of Hepatitis B Virus DNA in Liver Grafts Obtained from HBsAb and HBcAb Positive Organ Donors
- De Novo Superinfection of Hepatitis B Virus in an Anti-HBs Positive Patient with Recurrent Hepatitis C Following Liver Transplantation