Cancer Res Treat.
2008 Jun;40(2):53-61.
Cytoplasmic Trapping of CXCR4 in Hepatocellular Carcinoma Cell Lines
- Affiliations
-
- 1Division of Hematology/Oncology, Department of Internal Medicine, College of Medicine, Chungnam National University, Daejeon, Korea. deogyeon@cnu.ac.kr
Abstract
-
PURPOSE: The chemokine receptor CXCR4 plays a role in the metastasis and progression of a broad range of malignant tumors; however, its influence on hepatocellular carcinoma (HCC) is not well defined. Thus, we analyzed the expression of CXCR4 and its functions in HCC cell lines in vitro.
MATERIALS AND METHODS
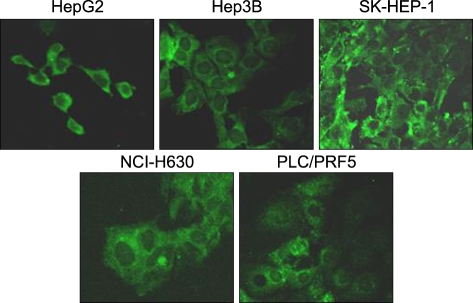
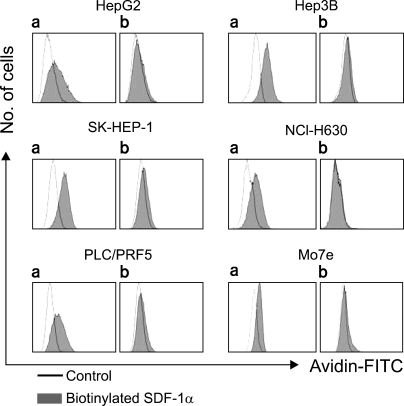
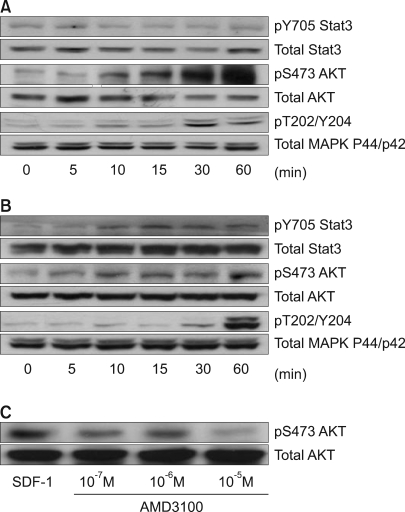
Five HCC cell lines (HepG2, Hep3B, SK-HEP-1, NCI-H630 and PLC/PRF5) were investigated. The CXCR4 expression was analyzed by RT-PCR, Western blotting, flow cytometry and immunofluorescence staining. In addition, the effects of stromal cell-derived factor-1 (SDF-1) on the migration, proliferation and survival of the cells were investigated, as well as the SDF-1-induced phosphorylation of signaling molecules.
RESULTS
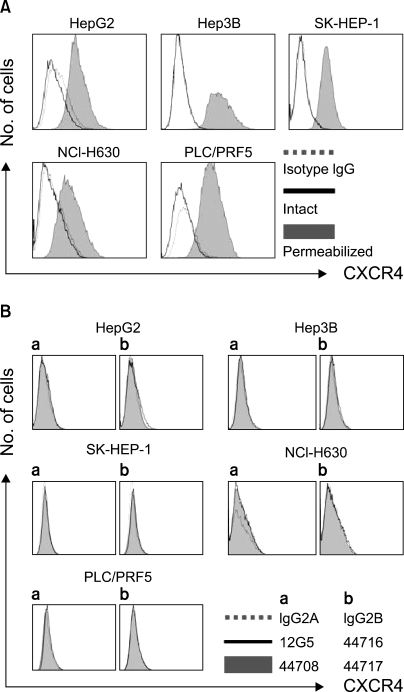
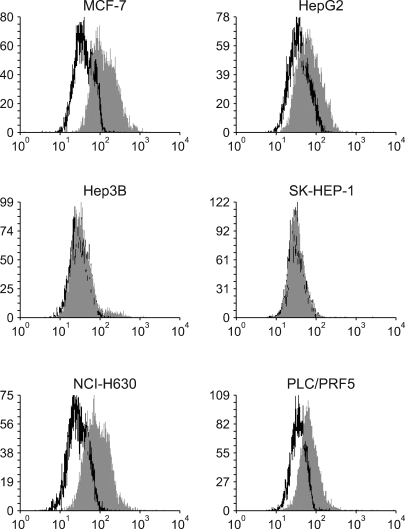
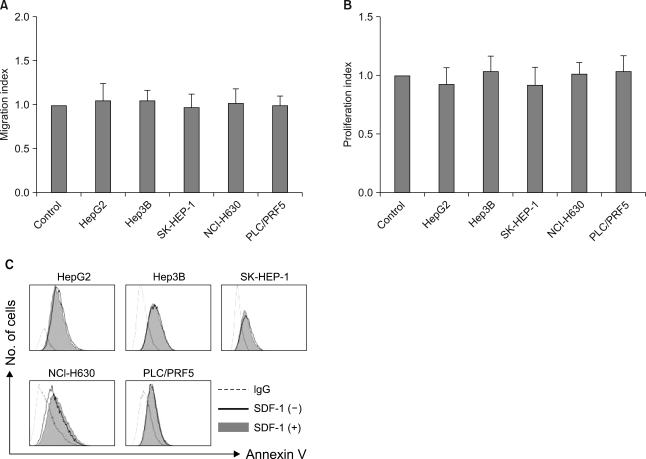
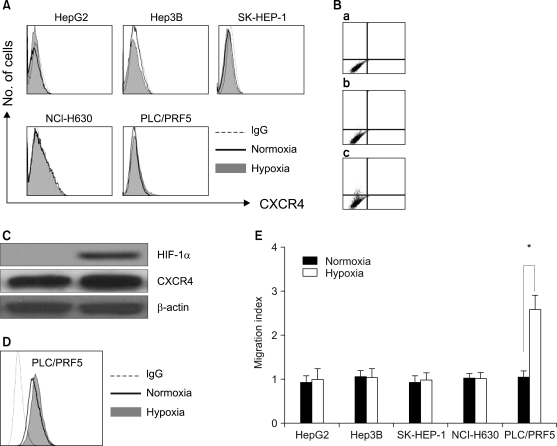
All five cell lines had abundant CXCR4 in their cytoplasm, whereas a cell surface CXCR4 expression was only detected in a very small population of PLC/ PRF5 cells. In contrast, SDF-1 bound to all the cells. SDF-1 induced the phosphorylation of AKT and ERK1/2 in the PLC/PRF5 cells and the phosphorylation of Stat3, AKT and ERK1/2 in the Hep3B cells. Nonetheless, SDF-1 did not induce migration or proliferation in any of the cells, nor did it rescue the cells from serum deprivation-induced apoptosis. Recruitment of CXCR4 from the cytoplasm to the cell surface was not elicited by dexamethasone, proinflammatory cytokines or VEGF. Hypoxia increased both the cytoplasmic and cell surface expressions of CXCR4 in only the PLC/PRF5 cells.
CONCLUSIONS
CXCR4 is trapped in the cytoplasm and it is not recruited to the cell surface by standard extrinsic stimuli in the majority of HCC cell lines, and the result of this is a negligible response to SDF-1.
MeSH Terms
Figure
Reference
-
1. Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999; 283:845–848. PMID: 9933168.
Article2. Altenburg JD, Broxmeyer HE, Jin Q, Cooper S, Basu S, Alkhatib G. A naturally occurring splice variant of CXCL12/stromal cell-derived factor 1 is a potent human immunodeficiency virus type 1 inhibitor with weak chemotaxis and cell survival activities. J Virol. 2007; 81:8140–8148. PMID: 17507482.
Article3. Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000; 6:3530–3535. PMID: 10999740.4. Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, et al. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002; 62:6304–6311. PMID: 12414661.5. Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008; 283:4283–4294. PMID: 18057003.
Article6. Zannettino AC, Farrugia AN, Kortesidis A, Manavis J, To LB, Martin SK, et al. Elevated serum levels of stromal-derived factor-1alpha are associated with increased osteoclast activity and osteolytic bone disease in multiple myeloma patients. Cancer Res. 2005; 65:1700–1709. PMID: 15753365.7. Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004; 18:1240–1242. PMID: 15180966.
Article8. Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, et al. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004; 64:8420–8427. PMID: 15548713.
Article9. Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004; 64:8604–8612. PMID: 15574767.
Article10. Guleng B, Tateishi K, Ohta M, Kanai F, Jazag A, Ijichi H, et al. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res. 2005; 65:5864–5871. PMID: 15994964.
Article11. Mitra P, De A, Ethier MF, Mimori K, Kodys K, Shibuta K, et al. Loss of chemokine SDF-1alpha-mediated CXCR4 signalling and receptor internalization in human hepatoma cell line HepG2. Cell Signal. 2001; 13:311–319. PMID: 11369512.12. Chu H, Zhou H, Liu Y, Liu X, Hu Y, Zhang J. Functional expression of CXC chemokine recepter-4 mediates the secretion of matrix metalloproteinases from mouse hepatocarcinoma cell lines with different lymphatic metastasis ability. Int J Biochem Cell Biol. 2007; 39:197–205. PMID: 16973405.
Article13. Sutton A, Friand V, Brule-Donneger S, Chaigneau T, Ziol M, Sainte-Catherine O, et al. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res. 2007; 5:21–33. PMID: 17259344.
Article14. Schimanski CC, Bahre R, Gockel I, Muller A, Frerichs K, Horner V, et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006; 95:210–217. PMID: 16819541.
Article15. Begum NA, Shibuta K, Mori M, Barnard GF. Reduced expression of the CXCR4 receptor mRNA in hepatocellular carcinoma and lack of inducibility of its ligand alpha-chemokine hIRH/SDF1alpha/PBSF in vitro. Int J Oncol. 1999; 14:927–934. PMID: 10200343.
Article16. Shibuta K, Mori M, Shimoda K, Inoue H, Mitra P, Barnard GF. Regional expression of CXCL12/CXCR4 in liver and hepatocellular carcinoma and cell-cycle variation during in vitro differentiation. Jpn J Cancer Res. 2002; 93:789–797. PMID: 12149145.17. Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006; 203:2201–2213. PMID: 16940167.
Article18. Signoret N, Oldridge J, Pelchen-Matthews A, Klasse PJ, Tran T, Brass LF, et al. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol. 1997; 139:651–664. PMID: 9348282.
Article19. Bruhl H, Cohen CD, Linder S, Kretzler M, Schlondorff D, Mack M. Post-translational and cell type-specific regulation of CXCR4 expression by cytokines. Eur J Immunol. 2003; 33:3028–3037. PMID: 14579271.20. Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005; 280:35760–35766. PMID: 16107333.
Article21. Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008; 205:479–490. PMID: 18268039.22. Hamon M, Mbemba E, Charnaux N, Slimani H, Brule S, Saffar L, et al. A syndecan-4/CXCR4 complex expressed on human primary lymphocytes and macrophages and HeLa cell line binds the CXC chemokine stromal cell-derived factor-1 (SDF-1). Glycobiology. 2004; 14:311–323. PMID: 15033938.
Article23. Wang J, Harada A, Matsushita S, Matsumi S, Zhang Y, Shioda T, et al. IL-4 and a glucocorticoid up-regulate CXCR4 expression on human CD4+ T lymphocytes and enhance HIV-1 replication. J Leukoc Biol. 1998; 64:642–649. PMID: 9823770.
Article24. Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998; 273:4282–4287. PMID: 9461627.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of CXCR4 Expression with Metastasis and Survival among Patients with Non-small Cell Lung Cancer
- Biology of SNU Cell Lines
- Study on the effect of various factors for the growth of human hepatocellular carcinoma, hepatoblastoma cell lines
- Analysis of Cellular Changes Resulting from Forced Expression of Dickkopf-1 in Hepatocellular Carcinoma Cells
- p53 gene mutation in hepatocellular carcinoma from Korean patients and in established hepatocellular carcinoma cell lines