Cancer Res Treat.
2007 Mar;39(1):30-36.
Analysis of Cellular Changes Resulting from Forced Expression of Dickkopf-1 in Hepatocellular Carcinoma Cells
- Affiliations
-
- 1Department of Immunology, School of Medicine, Kyungpook National University, Daegu, Korea. ysung@knu.ac.kr
- 2Immunomodulation Research Center, University of Ulsan, Ulsan, Korea.
Abstract
-
PURPOSE: Recent studies have shown that Dickkopf-1 (DKK-1) is overexpressed in some tumors, including hepatocellular carcinoma. However, the role of increased DKK-1 in these tumors is not known. In this study, the DKK-1 expression in hepatocellular carcinoma (HCC) cell lines was evaluated and the effect of DKK-1 overexpression in HCC cell lines was studied.
MATERIALS AND METHODS
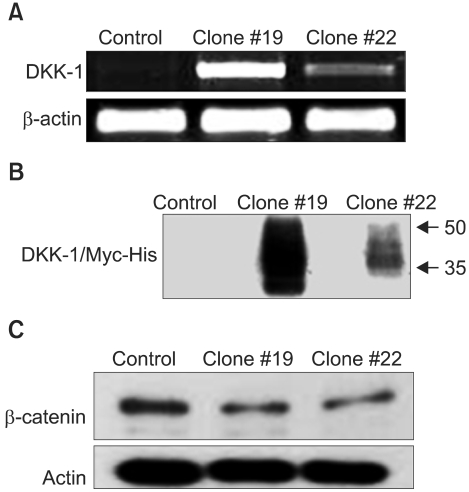
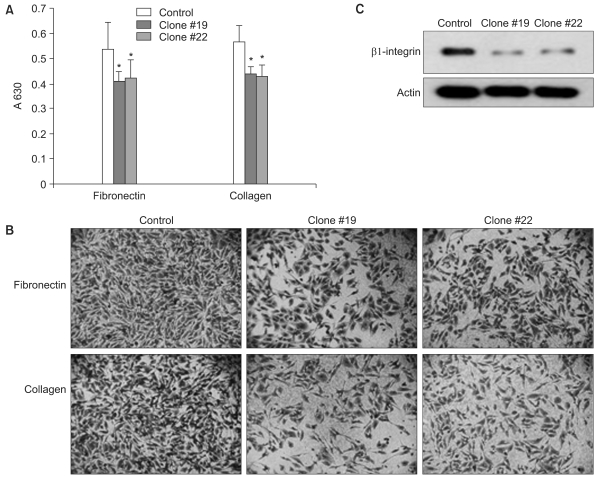
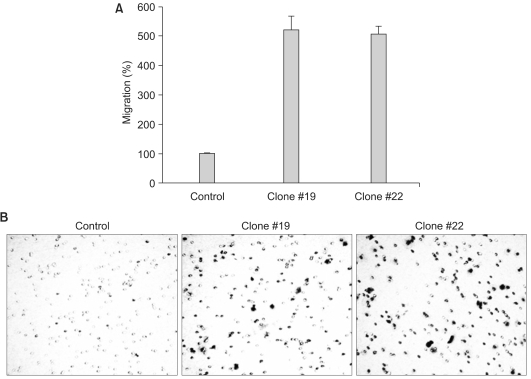
The expression of DKK-1 in hepatocellular carcinoma cell lines was evaluated by RT-PCR. Stable cell lines that overexpressed DKK-1 were established. Cell growth, adhesion, migration and invasion assays were performed.
RESULTS
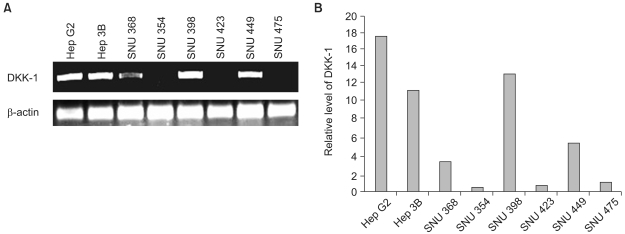
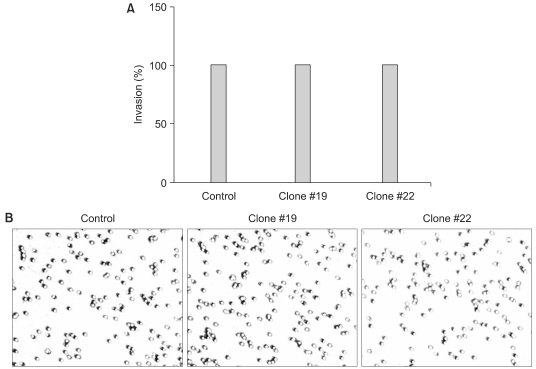
RT-PCR analysis showed that 5 out of 8 HCC cell lines expressed DKK-1. The forced expression of DKK-1 suppressed the growth of cells and increased the population of cells in the sub-G1 phase. In addition, DKK- 1 reduced the cellular adhesion capacity to collagen type I and fibronectin, and it increased migratory capacity. However, overexpression of DKK-1 did not increase the invasion capacity of the HCC cell line.
CONCLUSION
Collectively, our data suggest that overexpression of DKK-1 affects the biology of HCC cells.
MeSH Terms
Figure
Reference
-
1. Miler JR. The Wnts. Genome Biol. 2002; 3:REVIEWS3001. PMID: 11806834.2. Gonzalez-Sancho JM, Aguilera O, Garcia JM, Pendas-Franco N, Pena C, Cal S, et al. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005; 24:1098–1103. PMID: 15592505.3. Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999; 238:301–313. PMID: 10570958.
Article4. Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, et al. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004; 23:8520–8526. PMID: 15378020.5. Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, Espada J, et al. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006; 25:4116–4121. PMID: 16491118.
Article6. Lee AY, He B, You L, Xu Z, Mazieres J, Reguart N, et al. Dickkopf-1 antagonizes Wnt signaling independent of beta-catenin in human mesothelioma. Biochem Biophys Res Commun. 2004; 323:1246–1250. PMID: 15451431.7. Mikheev AM, Mikheeva SA, Liu B, Cohen P, Zarbl H. A functional genomics approach for the identification of putative tumor suppressor genes: Dickkopf-1 as suppressor of HeLa cell transformation. Carcinogenesis. 2004; 25:47–59. PMID: 14555616.
Article8. Wirths O, Waha A, Weggen S, Schirmacher P, Kuhne T, Goodyer CG, et al. Overexpression of human Dickkopf-1, an antagonist of wingless/WNT signaling, in human hepatoblastomas and Wilms' tumors. Lab Invest. 2003; 83:429–434. PMID: 12649343.
Article9. Patil MA, Chua M, Pan K, Lin R, Lih C, Cheung S, et al. An integrated data analysis approach to characterize genes highly expressed in hepatocellular carcinoma. Oncogene. 2005; 24:3737–3742. PMID: 15735714.
Article10. Park JG, Lee JH, Kang MS, Park KJ, Jeon YM, Lee HJ, et al. Characterization of cell lines established from human hepatocellular carcinoma. Int J Cancer. 1995; 62:276–282. PMID: 7543080.
Article11. Farooq M, Hwang SY, Park MK, Kim JC, Kim MK, Sung YK. Blocking endogenous glypican-3 expression releases Hep 3B cells from G1 arrest. Mol Cells. 2003; 15:356–360. PMID: 12872992.12. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998; 391:357–362. PMID: 9450748.
Article13. de la Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carci nomas. Proc Natl Acad Sci USA. 1998; 95:8847–8851. PMID: 9671767.14. Boyault S, Rickman DS, de Reynies A, Balabaud C, Rebouissou S, Jeannot E, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007; 45:42–52. PMID: 17187432.
Article15. Kondo Y, Kanai Y, Sakamoto M, Genda T, Mizokami M, Ueda R, et al. Beta-catenin accumulation and mutation of exon 3 of the beta-catenin gene in hepatocellular carcinoma. Jpn J Cancer Res. 1999; 90:1301–1309. PMID: 10665646.16. Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM, et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. 2002; 21:4863–4871. PMID: 12101426.17. Park JY, Park WS, Nam SW, Kim SY, Lee SH, Yoo NJ, et al. Mutations of beta-catenin and AXIN I genes are a late event in human hepatocellular carcinogenesis. Liver Int. 2005; 25:70–76. PMID: 15698401.18. Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, et al. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004; 127:1110–1122. PMID: 15480989.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cellular origin of liver cancer stem cells
- Kupffer Cells in Hepatocellular Carcinoma
- Collagen 24A1, a Potential Prognostic Biomarker in Hepatocellular Carcinoma
- Glutamine synthetase mediates sorafenib sensitivity in β-catenin-active hepatocellular carcinoma cells
- The Tissue Expression of HBsAg and HBcAg in Hepatocellular Carcinoma and Peritumoral Liver