Cancer Res Treat.
2010 Jun;42(2):82-94.
Time-course Transcriptional Profiling of Human Amniotic Fluid-derived Stem Cells Using Microarray
- Affiliations
-
- 1Department of Obstetrics and Gynecology, The Catholic University of Korea Colleg of Medicine, Seoul, Korea. ahnws@catholic.ac.kr
- 2Catholic Research Institutes of Medical Science, The Catholic University of Korea Colleg of Medicine, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, College of Medicine, Hanyang University, Seoul, Korea.
- 4Department of Biomedical Science, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 5Department of Plastic Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 6Department of Biotechnology, Seoul Women's University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
To maintain the homeostasis of stem cells and prevent their ability to initiate tumorigenesis, it is important to identify and modify factors that prevent or accelerate stem cell senescence. We used microarrays to attempt to identify such factors in human amniotic fluid (HAF)-derived stem cells.
MATERIALS AND METHODS
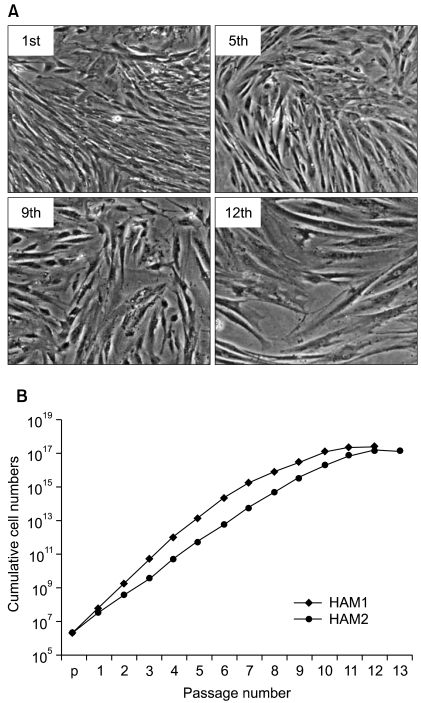
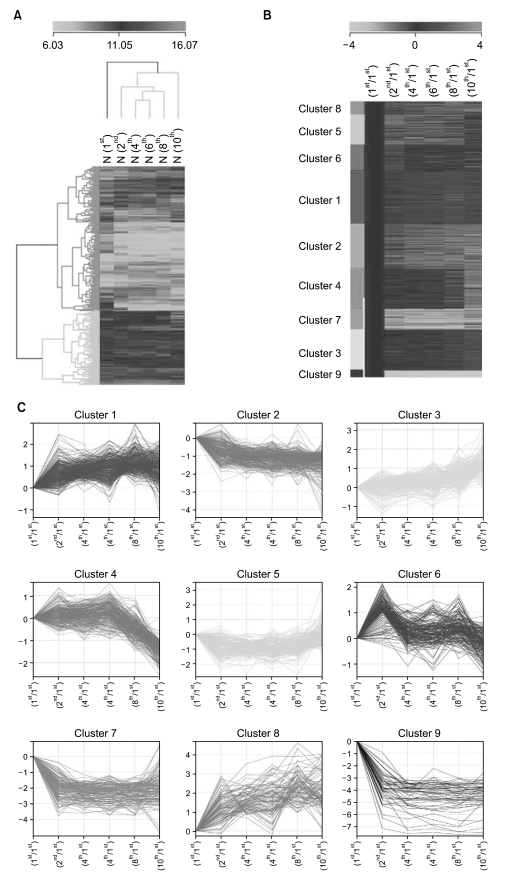
To identify gene expression changes over a time course, we compared gene expression profiles of HAF-derived stem cells in different passages (1st, 2nd, 4th, 6th, 8th, and 10th) using a Sentrix Human illumina microarray.
RESULTS
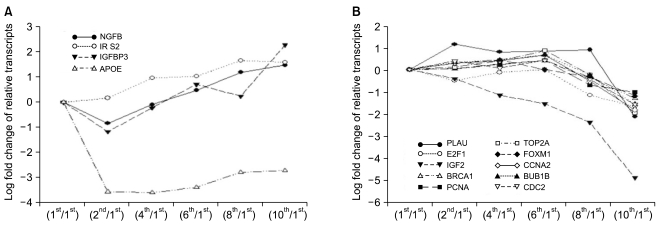
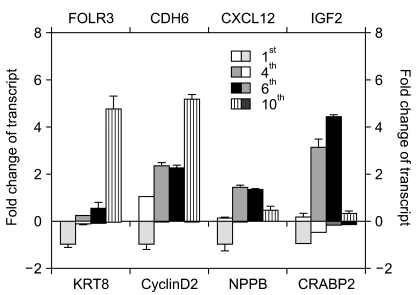
Of the 25,804 genes in the microarray chip, 1,970 showed an over 2-fold change relative to the control (the 1st passage)-either upregulated or downregulated. Quantitative real-time PCR validated the microarray data for selected genes: markedly increased genes were CXCL12, cadherin 6 (CDH6), and folate receptor 3 (FOLR3). Downregulated genes included cyclin D2, keratin 8, insulin-like growth factor 2 (IGF2), natriuretic peptide precursor B (NPPB) and cellular retinoic acid binding protein 2 (CRABP2). The expression pattern of the selected genes was consistent with the microarray data except for CXCL12 and IGF2. Interestingly, the expression of NPPB was dramatically downregulated along the time course; it was almost completely shut-down by the 10th passage. In contrast, FOLR3 mRNA expression was dramatically increased.
CONCLUSION
Taken together, although a function for NPPB and FOLR3 in stem cell senescence has not been reported, our results strongly suggest that NPPB and/or FOLR3 play a significant role in the regulation of stem cell senescence.
Keyword
MeSH Terms
-
Aging
Amniotic Fluid
Carrier Proteins
Cell Transformation, Neoplastic
Cyclin D2
Female
Folic Acid
Gene Expression
Homeostasis
Humans
Keratin-8
Nitrobenzoates
Real-Time Polymerase Chain Reaction
RNA, Messenger
Stem Cells
Transcriptome
Tretinoin
Carrier Proteins
Cyclin D2
Folic Acid
Keratin-8
Nitrobenzoates
RNA, Messenger
Tretinoin
Figure
Reference
-
1. Gosden CM. Amniotic fluid cell types and culture. Br Med Bull. 1983; 39:348–354. PMID: 6357346.
Article2. Milunsky A. Milunsky A, editor. Amniotic fluid cell culture. Genetic disorder and the fetus. 1979. New York: Plenum Press;p. 75–84.
Article3. Prusa AR, Marton E, Rosener M, Bernaschek G, Hengstschläger M. Oct-4 expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod. 2003; 18:1489–1493. PMID: 12832377.4. Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004; 19:1450–1456. PMID: 15105397.
Article5. Jacob J, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and polycomb-group gene bmi1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999; 397:164–168. PMID: 9923679.
Article6. Bauer JH, Poon PC, Glatt-Deeley H, Abrams JM, Helfand SL. Neuronal expression of p53 dominant-negative proteins in adult Drosophila melanogaster extends life span. Curr Biol. 2005; 15:2063–2068. PMID: 16303568.
Article7. Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002; 415:45–53. PMID: 11780111.
Article8. Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994; 211:90–98. PMID: 8125163.
Article9. Bond J, Jones C, Haughton M, DeMicco C, Kipling D, Wynford-Thomas D. Direct evidence from siRNA-directed "knock down" that p16 (INK4a) is required for human fibroblast senescence and for limiting ras-induced epithelial cell proliferation. Exp Cell Res. 2004; 292:151–156. PMID: 14720514.10. Kim J, Lee Y, Kim H, Hwang KJ, Kwon HC, Kim SK, et al. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007; 40:75–90. PMID: 17227297.
Article11. Pelicci PG. Do tumor-suppressive mechanisms contribute to organism aging by inducing stem cell senescence? J Clin Invest. 2004; 113:4–7. PMID: 14702099.
Article12. Itahana K, Zou Y, Itahana Y, Martinez JL, Beausejour C, Jacobs JJ, et al. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 2003; 23:389–401. PMID: 12482990.
Article13. Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003; 423:302–305. PMID: 12714971.
Article14. Feng L, Lin T, Uranishi H, Gu W, Xu Y. Functional analysis of the roles of posttranslational modifications at the p53 C terminus in regulating p53 stability and activity. Mol Cell Biol. 2005; 25:5389–5395. PMID: 15964796.
Article15. Maehara K, Yamakoshi K, Ohtani N, Kubo Y, Takahashi A, Arase S, et al. Reduction of total E2F/DP activity induces senescence-like cell cycle arrest in cancer cells lacking functional pRb and p53. J Cell Biol. 2005; 168:553–560. PMID: 15716376.
Article16. Campisi J, Dimri GP, Hara E. Schneider E, Rowe J, editors. Control of replicative senescence. Handbook of the biology of aging. 1996. 4th ed. New York NY: Academic Press;p. 121–149.17. Gil EB, Malone Link E, Liu LX, Johnson CD, Lees JA. Regulation of the insulin-like developmental pathway of Caenorhabditis elegans by a homolog of the PTEN tumor suppressor gene. Proc Natl Acad Sci U S A. 1999; 96:2925–2930. PMID: 10077613.
Article18. Fu VX, Schwarze SR, Kenowski ML, Leblanc S, Svaren J, Jarrard DF. A loss of insulin-like growth factor-2 imprinting is modulated by CCCTC-binding factor down-regulation at senescence in human epithelial cells. J Biol Chem. 2004; 279:52218–52226. PMID: 15471867.
Article19. Si SP, Tsou HC, Lee X, Peacocke M. Effect of cellular senescence and retinoic acid on the expression of cellular retinoic acid binding proteins in skin fibroblasts. Exp Cell Res. 1995; 219:243–248. PMID: 7628539.
Article20. Donato LJ, Noy N. Suppression of mammary carcinoma growth by retinoic acid: proapoptotic genes are targets for retinoic acid receptor and cellular retinoic acid-binding protein II signaling. Cancer Res. 2005; 65:8193–8199. PMID: 16166294.
Article21. Burnett JC JR, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986; 231:1145–1147. PMID: 2935937.
Article22. Kane MA, Portillo RM, Elwood PC, Antony AC, Kolhouse JF. The influence of extracellular folate concentration on methotrexate uptake by human KB cells. Partial characterization of a membrane-associated methotrexate binding protein. J Biol Chem. 1986; 261:44–49. PMID: 3941083.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Mini Overview of Isolation, Characterization and Application of Amniotic Fluid Stem Cells
- Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells
- Neurogenic potentials of human amniotic fluid-derived stem cells according to expression levels of stem cell markers and ingredients of induction medium
- Effect of Amniotic Fluid Stem Cells and Amniotic Fluid Cells on the Wound Healing Process in a White Rat Model
- Isolation and Identification of Respiratory Cells from Human Amniotic Fluid