Allergy Asthma Immunol Res.
2016 Mar;8(2):124-131. 10.4168/aair.2016.8.2.124.
Mimotopes for Api g 5, a Relevant Cross-reactive Allergen, in the Celery-Mugwort-Birch-Spice Syndrome
- Affiliations
-
- 1Department of Comparative Medicine, Messerli Research Institute of the University of Veterinary Medicine Vienna, the Medical University of Vienna and the University of Vienna, Vienna, Austria. erika.jensenjarolim@meduniwien.ac.at
- 2Department of Pathophysiology and Allergy Research, Center for Pathophysiology, Infectiology and Immunology, Medical University of Vienna, Vienna, Austria.
- 3AllergyCare, Allergy Diagnosis and Study Center, Vienna, Austria.
- KMID: 2165928
- DOI: http://doi.org/10.4168/aair.2016.8.2.124
Abstract
- PURPOSE
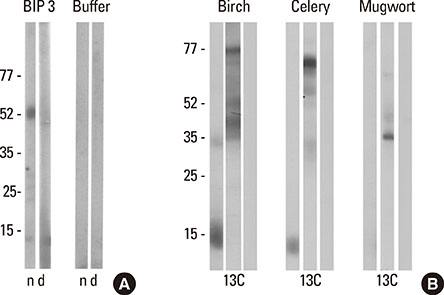
In the celery-mugwort-birch-spice syndrome, a significant proportion of IgE is directed against high molecular weight (HMW) glycoproteins, including the celery allergen Api g 5. BIP3, a monoclonal antibody originally raised against birch pollen, recognizes HMW allergens in birch and mugwort pollens, celery, and Apiaceae spices. Our aim was to generate mimotopes using BIP3 for immunization against the HMW allergens relevant in the celery-mugwort-birch-spice cross reactivity syndrome.
METHODS
Mimotopes were selected from a random-peptide display library by BIP3 and applied in IgE inhibition assays. The 3 phage clones with the highest inhibitory capacity were chosen for immunization of BALB/c mice. Mouse immune sera were tested for IgG binding to blotted birch pollen extract and used for inhibiting patients' IgE binding. Furthermore, sera were tested for binding to Api g 5, to horseradish peroxidase (HRP) as a second glycoprotein, or to non-glycosylated control allergen Phl p 5 in ELISA, and the specific Api g 5-specific IgG titers were determined.
RESULTS
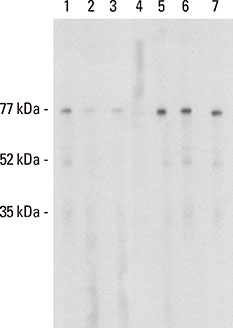
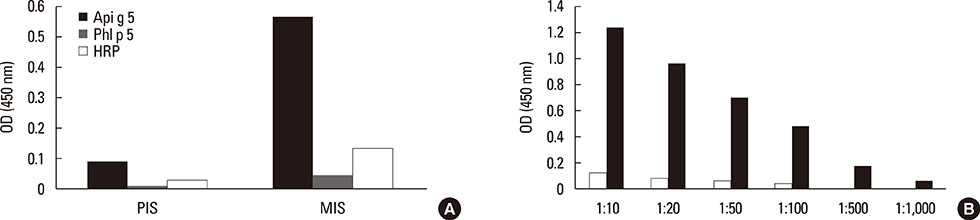
Three rounds of biopanning resulted in phage clones exhibiting 7 different sequences including 1 dominant, 1-6-cyclo-CHKLRCDKAIA. Three phage clones had the capacity to inhibit human IgE binding and induced IgG to the HMW antigen when used for immunizing BALB/c mice. The induced BIP3-mimotope IgG reached titers of 1:500 specifically to Api g 5, but hardly reacted to glycoprotein HRP, revealing a minor role of carbohydrates in their epitope.
CONCLUSIONS
The mimotopes characterized in this study mimic the epitope of BIP3 relevant for Api g 5, one of the cross-reactive HMW allergens relevant in the celery-mugwort-birch-spice syndrome. BIP3 mimotopes may be used in the future for hyposensitization in this clinical syndrome by virtue of good and specific immunogenicity.
Keyword
MeSH Terms
-
Allergens
Animals
Apiaceae
Apium graveolens
Artemisia
Bacteriophages
Betula
Carbohydrates
Clone Cells
Enzyme-Linked Immunosorbent Assay
Food Hypersensitivity
Glycoproteins
Horseradish Peroxidase
Humans
Immune Sera
Immunization
Immunoglobulin E
Immunoglobulin G
Mice
Molecular Weight
Pollen
Spices
Vaccination
Virtues
Allergens
Carbohydrates
Glycoproteins
Horseradish Peroxidase
Immune Sera
Immunoglobulin E
Immunoglobulin G
Figure
Reference
-
1. Jensen-Jarolim E, Leitner A, Hirschwehr R, Kraft D, Wüthrich B, Scheiner O, et al. Characterization of allergens in Apiaceae spices: anise, fennel, coriander and cumin. Clin Exp Allergy. 1997; 27:1299–1306.2. Vallier P, Dechamp C, Vial O, Deviller P. A study of allergens in celery with cross-sensitivity to mugwort and birch pollens. Clin Allergy. 1988; 18:491–500.3. Wüthrich B, Dietschi R. The celery-carrot-mugwort-condiment syndrome: skin test and RAST results. Schweiz Med Wochenschr. 1985; 115:258–264.4. Wüthrich B, Stäger J, Johansson SG. Celery allergy associated with birch and mugwort pollinosis. Allergy. 1990; 45:566–571.5. Jarolim E, Tejkl M, Rohac M, Schlerka G, Scheiner O, Kraft D, et al. Monoclonal antibodies against birch pollen allergens: characterization by immunoblotting and use for single-step affinity purification of the major allergen Bet v I. Int Arch Allergy Appl Immunol. 1989; 90:54–60.6. Fiocchi A, Dahdah L, Martelli A, Mazzina O, Manzotti G. Spice allergies in children. Ann Allergy Asthma Immunol. 2014; 112:72–73.7. Asero R. Reply to the letter 'multiple nonsteroidal anti-inflammatory drug-induced cutaneous disease: relevance, natural evolution and relationship with atopy' by blanca-lópez et Al. Int Arch Allergy Immunol. 2014; 164:149–150.8. André F, André C, Colin L, Cacaraci F, Cavagna S. Role of new allergens and of allergens consumption in the increased incidence of food sensitizations in France. Toxicology. 1994; 93:77–83.9. Asero R. Relevance of pollen-specific IgE levels to the development of Apiaceae hypersensitivity in patients with birch pollen allergy. Allergy. 1997; 52:560–564.10. Ballmer-Weber BK, Vieths S, Lüttkopf D, Heuschmann P, Wüthrich B. Celery allergy confirmed by double-blind, placebo-controlled food challenge: a clinical study in 32 subjects with a history of adverse reactions to celery root. J Allergy Clin Immunol. 2000; 106:373–378.11. Scheurer S, Wangorsch A, Haustein D, Vieths S. Cloning of the minor allergen Api g 4 profilin from celery (Apium graveolens) and its cross-reactivity with birch pollen profilin Bet v 2. Clin Exp Allergy. 2000; 30:962–971.12. Pastorello EA, Farioli L, Stafylaraki C, Scibilia J, Giuffrida MG, Mascheri A, et al. Fennel allergy is a lipid-transfer protein (LTP)-related food hypersensitivity associated with peach allergy. J Agric Food Chem. 2013; 61:740–746.13. Vejvar E, Himly M, Briza P, Eichhorn S, Ebner C, Hemmer W, et al. Allergenic relevance of nonspecific lipid transfer proteins 2: Identification and characterization of Api g 6 from celery tuber as representative of a novel IgE-binding protein family. Mol Nutr Food Res. 2013; 57:2061–2070.14. Dewitt AM, Andersson K, Peltre G, Lidholm J. Cloning, expression and immunological characterization of full-length timothy grass pollen allergen Phl p 4, a berberine bridge enzyme-like protein with homology to celery allergen Api g 5. Clin Exp Allergy. 2006; 36:77–86.15. Fötisch K, Altmann F, Haustein D, Vieths S. Involvement of carbohydrate epitopes in the IgE response of celery-allergic patients. Int Arch Allergy Immunol. 1999; 120:30–42.16. Borghesan F, Mistrello G, Amato S, Giuffrida MG, Villalta D, Asero R. Mugwort-fennel-allergy-syndrome associated with sensitization to an allergen homologous to Api g 5. Eur Ann Allergy Clin Immunol. 2013; 45:130–137.17. Bublin M, Radauer C, Wilson IB, Kraft D, Scheiner O, Breiteneder H, et al. Cross-reactive N-glycans of Api g 5, a high molecular weight glycoprotein allergen from celery, are required for immunoglobulin E binding and activation of effector cells from allergic patients. FASEB J. 2003; 17:1697–1699.18. Ganglberger E, Radauer C, Grimm R, Hoffmann-Sommergruber K, Breiteneder H, Scheiner O, et al. N-terminal sequences of high molecular weight allergens from celery tuber. Clin Exp Allergy. 2000; 30:566–570.19. Bublin M, Lauer I, Oberhuber C, Alessandri S, Briza P, Radauer C, et al. Production and characterization of an allergen panel for component-resolved diagnosis of celery allergy. Mol Nutr Food Res. 2008; 52:Suppl 2. S241–S250.20. Jensen-jarolim E, Leitner A, Kalchhauser H, Zürcher A, Ganglberger E, Bohle B, et al. Peptide mimotopes displayed by phage inhibit antibody binding to bet v 1, the major birch pollen allergen, and induce specific IgG response in mice. FASEB J. 1998; 12:1635–1642.21. Wuhrer M, Balog CI, Koeleman CA, Deelder AM, Hokke CH. New features of site-specific horseradish peroxidase (HRP) glycosylation uncovered by nano-LC-MS with repeated ion-isolation/fragmentation cycles. Biochim Biophys Acta. 2005; 1723:229–239.22. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254.23. Luzzago A, Felici F, Tramontano A, Pessi A, Cortese R. Mimicking of discontinuous epitopes by phage-displayed peptides, I. Epitope mapping of human H ferritin using a phage library of constrained peptides. Gene. 1993; 128:51–57.24. Aalberse RC, van Ree R. Crossreactive carbohydrate determinants. Clin Rev Allergy Immunol. 1997; 15:375–387.25. Ballmer-Weber BK, Hoffmann A, Wüthrich B, Lüttkopf D, Pompei C, Wangorsch A, et al. Influence of food processing on the allergenicity of celery: DBPCFC with celery spice and cooked celery in patients with celery allergy. Allergy. 2002; 57:228–235.26. Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int Arch Allergy Immunol. 2002; 129:286–295.27. Helbling A, Lopez M, Schwartz HJ, Lehrer SB. Reactivity of carrot-specific IgE antibodies with celery, apiaceous spices, and birch pollen. Ann Allergy. 1993; 70:495–499.28. Stäger J, Wüthrich B, Johansson SG. Spice allergy in celery-sensitive patients. Allergy. 1991; 46:475–478.29. Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007; 142:99–115.30. Mari A, Iacovacci P, Afferni C, Barletta B, Tinghino R, Di Felice G, et al. Specific IgE to cross-reactive carbohydrate determinants strongly affect the in vitro diagnosis of allergic diseases. J Allergy Clin Immunol. 1999; 103:1005–1011.31. van der Veen MJ, van Ree R, Aalberse RC, Akkerdaas J, Koppelman SJ, Jansen HM, et al. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997; 100:327–334.32. Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008; 358:1109–1117.33. Commins SP, Platts-Mills TA. Anaphylaxis syndromes related to a new mammalian cross-reactive carbohydrate determinant. J Allergy Clin Immunol. 2009; 124:652–657.34. Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007; 364:8–18.35. Foetisch K, Westphal S, Lauer I, Retzek M, Altmann F, Kolarich D, et al. Biological activity of IgE specific for cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2003; 111:889–896.36. Jin C, Hantusch B, Hemmer W, Stadlmann J, Altmann F. Affinity of IgE and IgG against cross-reactive carbohydrate determinants on plant and insect glycoproteins. J Allergy Clin Immunol. 2008; 121:185–190.e2.37. Schöll I, Wiedermann U, Förster-Waldl E, Ganglberger E, Baier K, Boltz-Nitulescu G, et al. Phage-displayed Bet mim 1, a mimotope of the major birch pollen allergen Bet v 1, induces B cell responses to the natural antigen using bystander T cell help. Clin Exp Allergy. 2002; 32:1583–1588.38. Wallmann J, Epstein MM, Singh P, Brunner R, Szalai K, El-Housseiny L, et al. Mimotope vaccination for therapy of allergic asthma: anti-inflammatory effects in a mouse model. Clin Exp Allergy. 2010; 40:650–658.39. Pacios LF, Tordesillas L, Cuesta-Herranz J, Compes E, Sánchez-Monge R, Palacín A, et al. Mimotope mapping as a complementary strategy to define allergen IgE-epitopes: peach Pru p 3 allergen as a model. Mol Immunol. 2008; 45:2269–2276.40. Bauermeister K, Ballmer-Weber BK, Bublin M, Fritsche P, Hanschmann KM, Hoffmann-Sommergruber K, et al. Assessment of component-resolved in vitro diagnosis of celeriac allergy. J Allergy Clin Immunol. 2009; 124:1273–1281.e2.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Are there any links between mugwort pollen and food allergens such as celery and carrot based upon allergy skin prick tests?
- Chinese Bellflower Root Anaphylaxis: IgE-Binding Components and Cross-Reactivity With Mugwort and Birch
- Oral allergy syndrome associated with weed pollinosis
- Comparison of Component-Resolved Diagnosis by Using Allergen Microarray With the Conventional Tests in Allergic Rhinitis Patients: The First Using in Korea
- Standardization of Weed Pollen Extracts, Japanese Hop and Mugwort, in Korea