Brain Neurorehabil.
2015 Sep;8(2):90-95. 10.12786/bn.2015.8.2.90.
Application of Non-invasive Brain Stimulation for Neurorehabilitation: Cerebellar Stimulation
- Affiliations
-
- 1Department of Rehabilitation Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Korea. njpaik@snu.ac.kr
- KMID: 2165196
- DOI: http://doi.org/10.12786/bn.2015.8.2.90
Abstract
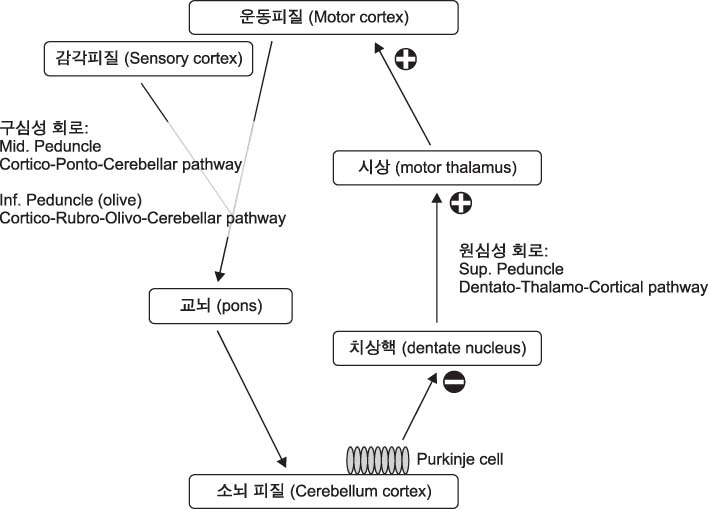
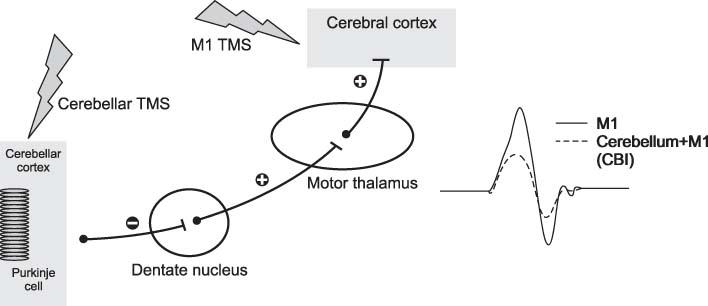
- Cerebellum serves an important function in diverse domain of motor, cognition control. Cerebellar non-invasive brain stimulation (NIBS) can provide a better comprehension of cerebellar circuity connecting to primary motor cortex. Cerebellar transcranial magnetic stimulation (TMS) activates Purkinje cells, causing increased inhibition of dentato-thalamo-cortical pathway. Assessing cerebellar-brain inhibition is useful for evaluating normal cerebellar functions and for understanding specific pathophysiology. Transcranial direct current stimulation (tDCS) has the polarity specific effect on cerebellar activity. Both TMS and tDCS can modulate cerebellar functions: motor learning, visuomotor adaptation, motor coordination, working memory and other cognitive domains. Further studies are encouraged to accumulate clinical and molecular evidences of neural plasticity induced by cerebellar NIBS. In the near future, cerebellar NIBS would play a crucial role in the field of neurorehabiliation.
Keyword
MeSH Terms
Figure
Reference
-
1. Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, et al. Non-invasive cerebellar stimulation--a consensus paper. Cerebellum. 2014; 13:121–138.2. Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995; 37:703–713.3. Jayaram G, Galea JM, Bastian AJ, Celnik P. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex. 2011; 21:1901–1909.4. Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009; 29:9115–9122.5. Shah B, Nguyen TT, Madhavan S. Polarity independent effects of cerebellar tdcs on short term ankle visuomotor learning. Brain Stimul. 2013; 6:966–968.6. Ferrucci R, Brunoni AR, Parazzini M, Vergari M, Rossi E, Fumagalli M, et al. Modulating human procedural learning by cerebellar transcranial direct current stimulation. Cerebellum. 2013; 12:485–492.7. Grimaldi G, Argyropoulos GP, Bastian A, Cortes M, Davis NJ, Edwards DJ, et al. Cerebellar transcranial direct current stimulation (ctdcs): A novel approach to understanding cerebellar function in health and disease. Neuroscientist. 2014; 11. 18. pii: 1073858414559409. [Epub ahead of print].8. Jayaram G, Tang B, Pallegadda R, Vasudevan EV, Celnik P, Bastian A. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol. 2012; 107:2950–2957.9. Galea JM, Vazquez A, Pasricha N, de Xivry JJ, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: The motor cortex retains what the cerebellum learns. Cereb Cortex. 2011; 21:1761–1770.10. Desmond JE, Chen SH, Shieh PB. Cerebellar transcranial magnetic stimulation impairs verbal working memory. Ann Neurol. 2005; 58:553–560.11. Silveri MC, Di Betta AM, Filippini V, Leggio MG, Molinari M. Verbal short-term store-rehearsal system and the cerebellum. Evidence from a patient with a right cerebellar lesion. Brain. 1998; 121(Pt 11):2175–2187.
Article12. Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional mri. J Neurosci. 1997; 17:9675–9685.
Article13. Pope PA, Miall RC. Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimul. 2012; 5:84–89.
Article14. Hiraoka K, Horino K, Yagura A, Matsugi A. Cerebellar tms evokes a long latency motor response in the hand during a visually guided manual tracking task. Cerebellum. 2010; 9:454–460.
Article15. Torriero S, Oliveri M, Koch G, Caltagirone C, Petrosini L. Interference of left and right cerebellar rtms with procedural learning. J Cogn Neurosci. 2004; 16:1605–1611.
Article16. Argyropoulos GP, Muggleton NG. Effects of cerebellar stimulation on processing semantic associations. Cerebellum. 2013; 12:83–96.
Article17. Lesage E, Morgan BE, Olson AC, Meyer AS, Miall RC. Cerebellar rtms disrupts predictive language processing. Curr Biol. 2012; 22:R794–R795.
Article18. Ugawa Y, Genba-Shimizu K, Rothwell JC, Iwata M, Kanazawa I. Suppression of motor cortical excitability by electrical stimulation over the cerebellum in ataxia. Ann Neurol. 1994; 36:90–96.
Article19. Ugawa Y, Terao Y, Hanajima R, Sakai K, Furubayashi T, Machii K, et al. Magnetic stimulation over the cerebellum in patients with ataxia. Electroencephalogr Clin Neurophysiol. 1997; 104:453–458.
Article20. Shirota Y, Hamada M, Hanajima R, Terao Y, Matsumoto H, Ohminami S, et al. Cerebellar dysfunction in progressive supranuclear palsy: A transcranial magnetic stimulation study. Mov Disord. 2010; 25:2413–2419.
Article21. Lee SA, Oh BM, Kim SJ, Paik NJ. The molecular evidence of neural plasticity induced by cerebellar repetitive transcranial magnetic stimulation in the rat brain: A preliminary report. Neurosci Lett. 2014; 575:47–52.
Article22. Shimizu H, Tsuda T, Shiga Y, Miyazawa K, Onodera Y, Matsuzaki M, et al. Therapeutic efficacy of transcranial magnetic stimulation for hereditary spinocerebellar degeneration. Tohoku J Exp Med. 1999; 189:203–211.
Article23. Shiga Y, Tsuda T, Itoyama Y, Shimizu H, Miyazawa KI, Jin K, et al. Transcranial magnetic stimulation alleviates truncal ataxia in spinocerebellar degeneration. J Neurol Neurosurg Psychiatry. 2002; 72:124–126.
Article24. Kim WS, Jung SH, Oh MK, Min YS, Lim JY, Paik NJ. Effect of repetitive transcranial magnetic stimulation over the cerebellum on patients with ataxia after posterior circulation stroke: A pilot study. J Rehabil Med. 2014; 46:418–423.
Article25. Bonni S, Ponzo V, Caltagirone C, Koch G. Cerebellar theta burst stimulation in stroke patients with ataxia. Funct Neurol. 2014; 29(1):41–45.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuromodulation and Brain Plasticity
- Application of Non-invasive Brain Stimulation on Dysphagia after Stroke
- Novel Approaches of Non-Invasive Stimulation Techniques to Motor Rehabilitation Following Stroke: A Review
- Safety of Transcranial Direct Current Stimulation in Neurorehabilitation
- Enhancing Motor Learning with Transcranial Direct Current Stimulation