J Vet Sci.
2015 Jun;16(2):165-171. 10.4142/jvs.2015.16.2.165.
Micro-computed tomography evaluation and pathological analyses of female rats with collagen-induced arthritis
- Affiliations
-
- 1Department of Biomedical Laboratory Science, Namseoul University, Cheonan 330-707, Korea. kang@nsu.ac.kr
- KMID: 2164525
- DOI: http://doi.org/10.4142/jvs.2015.16.2.165
Abstract
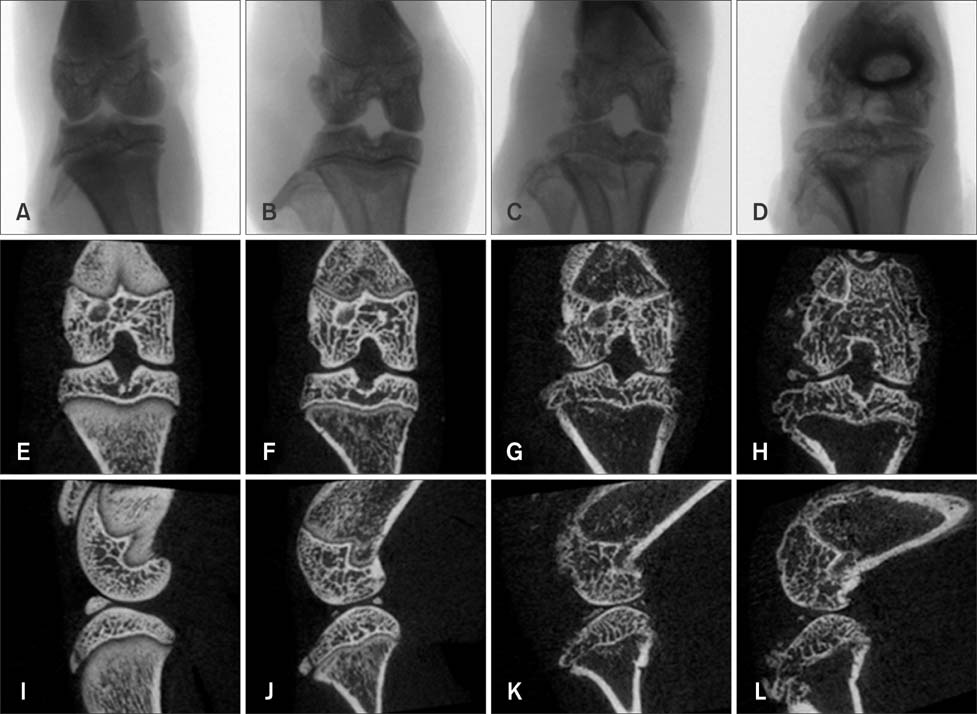
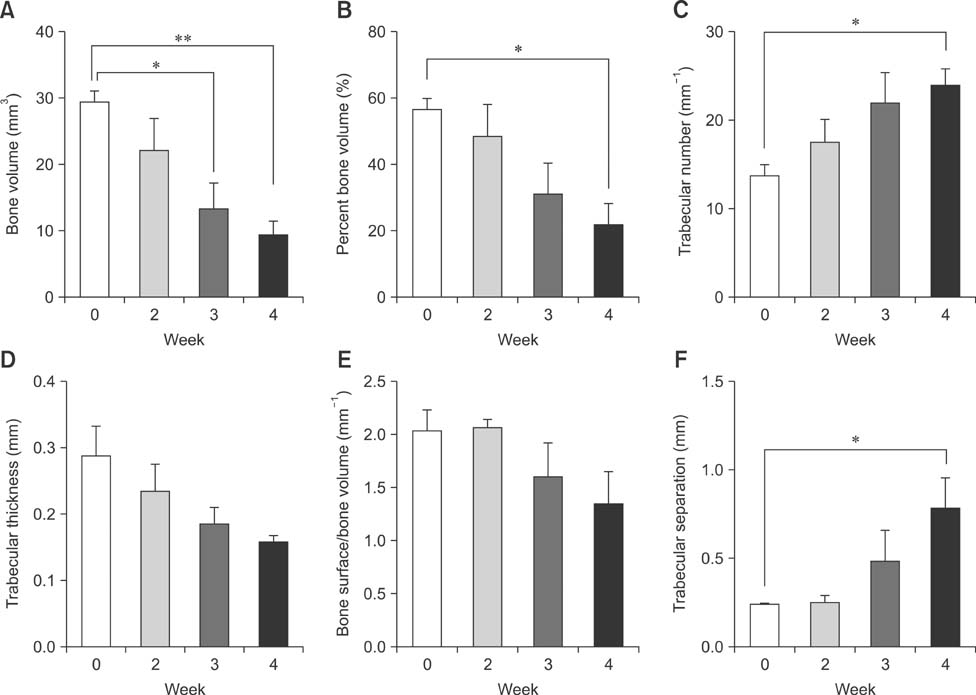
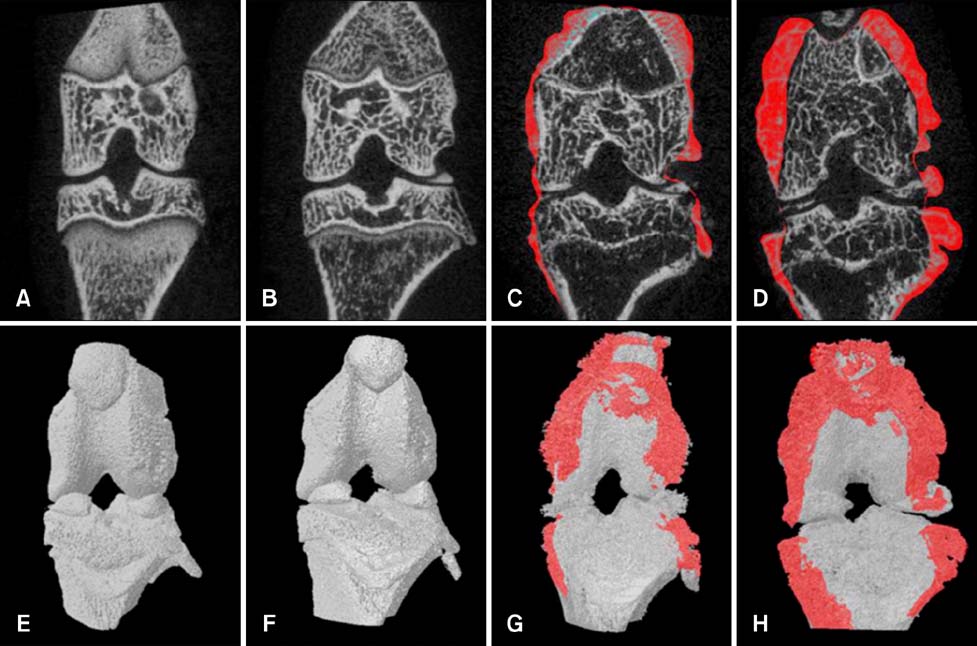
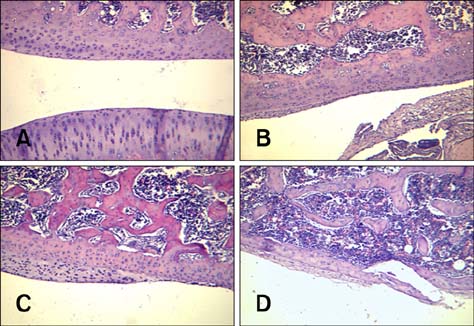
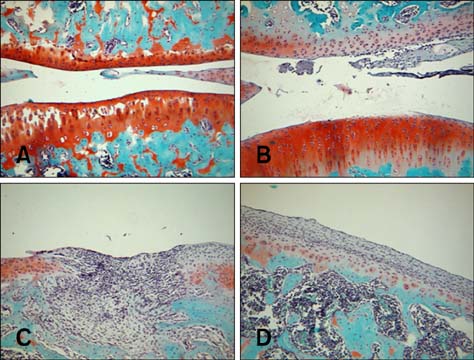
- Imaging techniques have been introduced to assess the efficacy and toxicity of developing pharmaceuticals. The purpose of this study was to perform a comprehensive characterization of collagen-induced arthritis (CIA) in rats using micro-computed tomography (micro-CT) and to compare the results with data from conventional pathological examination. Arthritis was induced by collagen in 24 female Wistar rats. Micro-CT and pathological analyses were performed to assess arthritis progression. Micro-CT analysis showed marked joint destruction occurring in a time-dependent manner following collagen administration. Bone volume was significantly decreased in the tibia at weeks 3 and 4 compared to week 0 (p < 0.05 and p < 0.01, respectively). Additionally, percent bone volume was significantly reduced in the tibia at week 4 compared to week 0 (p < 0.05). In contrast, bone surface/bone volume and trabecular separation were significantly increased in the tibia of the animals at week 4 compared to week 0 (p < 0.05). Severe joint destruction with extensive inflammation, erosion of cartilage and bone, and infiltration of inflammatory cells were observed in the knee joints of the collagen-treated rats. Taken together, micro-CT made it possible to quantify CIA lesions and should be performed with pathological examination in rats.
Keyword
MeSH Terms
Figure
Reference
-
1. Batiste DL, Kirkley A, Laverty S, Thain LMF, Spouge AR, Holdsworth DW. Ex vivo characterization of articular cartilage and bone lesions in a rabbit ACL transection model of osteoarthritis using MRI and micro-CT. Osteoarthritis Cartilage. 2004; 12:986–996.
Article2. Bendele A. Animal models of rheumatoid arthritis. J Musculoskelet Neuronal Interact. 2001; 1:377–385.3. Bevaart L, Vervoordeldonk MJ, Tak PP. Evaluation of therapeutic targets in animal models of arthritis: how does it relate to rheumatoid arthritis? Arthritis Rheum. 2010; 62:2192–2205.
Article4. Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007; 2:1269–1275.
Article5. Carvalheiro H, da Silva JAP, Souto-Carneiro MM. Potential roles for CD8+ T cells in rheumatoid arthritis. Autoimmun Rev. 2013; 12:401–409.6. de Jong M, Essers J, van Weerden WM. Imaging preclinical tumour models: improving translational power. Nat Rev Cancer. 2014; 14:481–493.
Article7. Ellenbroek SI, van Rheenen J. Imaging hallmarks of cancer in living mice. Nat Rev Cancer. 2014; 14:406–418.
Article8. Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010; 18:Suppl 3. S24–S34.
Article9. Goebel JC, Bolbos R, Pham M, Galois L, Rengle A, Loeuille D, Netter P, Gillet P, Beuf O, Watrin-Pinzano A. In vivo high-resolution MRI (7T) of femoro-tibial cartilage changes in the rat anterior cruciate ligament transection model of osteoarthritis: a cross-sectional study. Rheumatology (Oxford). 2010; 49:1654–1664.
Article10. Ishikawa T, Nishigaki F, Miyata S, Hirayama Y, Minoura K, Imanishi J, Neya M, Mizutani T, Imamura Y, Naritomi Y, Murai H, Ohkubo Y, Kagayama A, Mutoh S. Prevention of progressive joint destruction in collagen-induced arthritis in rats by a novel matrix metalloproteinase inhibitor, FR255031. Br J Pharmacol. 2005; 144:133–143.
Article11. Knoerzer DB, Donovan MG, Schwartz BD, Mengle-Gaw LJ. Clinical and histological assessment of collagen-induced arthritis progression in the diabetes-resistant BB/Wor rat. Toxicol Pathol. 1997; 25:13–19.
Article12. Koba W, Jelicks LA, Fine EJ. MicroPET/SPECT/CT imaging of small animal models of disease. Am J Pathol. 2013; 182:319–324.
Article13. Maruotti N, Cantatore FP, Crivellato E, Vacca A, Ribatti D. Macrophages in rheumatoid arthritis. Histol Histopathol. 2007; 22:581–586.14. Myers LK, Rosloniec EF, Cremer MA, Kang AH. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997; 61:1861–1878.
Article15. Otero M, Goldring MB. Cells of the synovium in rheumatoid arthritis. Chondrocytes. Arthritis Res Ther. 2007; 9:220.
Article16. Ryu JH, Lee A, Chu JU, Koo H, Ko CY, Kim HS, Yoon SY, Kim BS, Choi K, Kwon IC, Kim K, Youn I. Early diagnosis of arthritis in mice with collagen-induced arthritis, using a fluorogenic matrix metalloproteinase 3-specific polymeric probe. Arthritis Rheum. 2011; 63:3824–3832.
Article17. Schambach SJ, Bag S, Schilling L, Groden C, Brockmann MA. Application of micro-CT in small animal imaging. Methods. 2010; 50:2–13.
Article18. Scott DL. Biologics-based therapy for the treatment of rheumatoid arthritis. Clin Pharmacol Ther. 2012; 91:30–43.
Article19. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010; 376:1094–1108.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Methotrexate on Collagen-Induced Arthritis Assessed by Micro-Computed Tomography and Histopathological Examination in Female Rats
- Fluorescence bioimaging analysis of collagen antibody-induced arthritis in male mice
- Assessment of collagen antibody-induced arthritis in BALB/c mice using bioimaging analysis and histopathological examination
- The evaluation of the correlation between histomorphometric analysis and micro-computed tomography analysis in AdBMP-2 induced bone regeneration in rat calvarial defects
- The Effect of Long Chain N-3 Polyunsaturated Fatty Acids on Development of Collagen-induced Arthritis in Rats